


Continued advances in angiographic imaging, especially flat panel DYNA variety we use, allow for tremendously better visualization. This goes hand in hand with our evolving understanding. The old ophthalmic artery page may be found here. See Diagrams and Drawings page for keys to figures below.


This comprehensive review will include basic embryology, various sources of ophthalmic artery/ orbital supply, review of intrinsic / orbital ophthalmic vasculature, and specifically supply to the optic nerve / central retinal artery.
The main ideas here, and everywhere in vascular anatomy, are balance and spectrum. There is balance among different sources of supply to the same organ/tissue, which can be altered by physiology or disease. There is a continuous spectrum of variation in this supply — more balanced arrangements are more common, and progressively unbalanced ones are correspondingly uncommon. The ophthalmic system very much follows this.
There are multiple sources of supply to the orbit, mainly from ophthalmic and middle meningeal arteries. Their relative contributions are in balance. The various sites of ophthalmic artery origin (classic, dorsal, ventral, etc.) are also part of a spectrum. The orbit is, of course, an extracranial, facial structure. Thus, the amount of arterial supply to its contents is virtually inexhaustible. However, within the orbit there is a unique extension of the brain — the optic nerve and parts of globe. Its vascular supply, exemplified by the central retinal artery, is both unique and characteristically less redundant, as is typical for brain. Although CRA is not a true end-artery, its collateral supply is extremely limited. Advances in imaging allow for a more complete understanding of this.
Embryology
Embryology is important. Discussions of ophthalmic artery embryology also span a spectrum — from reasonable to obscure to stages of bizzare. Our data is limited. There are two conflicting views — the Padget and Lasjaunias ones. For a taste of this kind of debate, see reference here and form your own opinion. The point here is to show that, despite limited knowledge, this embryology is not irrelevant. We just need to look at it from a different perspective — of course, it’s the Spectrum one.
The big picture is this: In early embryonic stages, there are several sources of supply to what will become the orbit, from various points along the intracranial carotid artery, its cranial ramus (future ACA), and the hyostapedial system that will develop in part into the MMA — see this page for more practical embryology info on that. This early supply appears to change with development. In most cases, the final usual origin of ophthalmic artery is from the very proximal intradural ICA, as in the image below (there are however to unusual things about this image — see below as we go…)

In some cases, however, the orbital supply with reflect persistence of more unusual and likely early embryonic sources — to be discussed below.
The so-called “dorsal ophthalmic” (letter B in diagram below) — ophthalmic artery origin is from horizontal cavernous segment, represents a hypertrophied anteromedial branch of the ILT — basically the anteromedial branch is the ophthalmic artery (entering orbit via superior orbital fissure). This is the Lasjaunias camp view — see images below and dedicated “Dorsal Ophthalmic Artery” page.


Although we focus on angiography, MR and CT are perfectly good enough to show a typical dorsal ophthalmic (red arrows). Here is an example from an MRI circa 2010 — old quality by now, but certainly good enough. “Classic” one is on right (yellow)

MIPs

The other classic origin, extremely rare, is the “ventral ophthalmic” (letter “A” above) — from the ACOM area or A1 segment — see images below of the several cases we encountered and a dedicated “Ventral Ophthalmic Artery” page.

Another example — arising from mid-A1 segment. See “Ventral Ophthalmic Artery” page for treatment of this aneurysm


Both “dorsal” and “ventral” refer to positions in the embryo, not adult, in case you are wondering, and have embryologic implications that are debatable — but you should know the names.
Much less discussed are more frequent and correspondingly less dramatic variations in location of ophthalmic artery origin along the paraophthalmic segment ICA — from “cave” type extradural to relatively distal “hypophyseal” type locations.
Below is an old, old image of extradural origin. We have plenty of new ones, but love history

So, how to put it together? There will be no kumbaya moment here. Spectrum is how we think of it — proximal intradural is most common, and points away from this site are progressively rarer. See below.

It is a simple and logical way, and we have no doubt that everyone will now stop arguing and think the way we do. Just kidding… but we hope this makes some sense to you, whatever the fine debatable points are.
Below is relatively distal origin of ophthalmic artery — at “mid-supraclinoid” or “mid-hypophyseal” segment — halfway between where it usually is and the PCOM — both sides same patient


Multiple intracranial origin sources of ophthalmic supply
Now, here is another consequence of having a spectrum — there does not have to be only one ophthalmic artery. Similar to duplicated MCAs, SCAs, AICAs, etc (not exactly but same general idea) there can, on occasion, persist dual intracranial supply to orbit — two ophthalmic arteries, if you will. Below is one example — purple is classic, red is dorsal.

Below is another one — blue is classic, red is dorsal, purple is a beautiful Bernasconi-Cassinari (artery of free margin). Nice examples of the ILT anteromedial branch idea. See how the anterolateral branch/dorsal ophthalmic enters via the superior orbital fissure, below the classic one?

Beautiful example of physiologic balance below — Pipeline embolization of the aneurysm is associated with rearrangement of orbital supply, with closure of the classic branch (as it should if we want the aneurysm to close), and exclusive orbital supply by the dorsal ophthalmic, which we did not cover. For the astute ones, the coil artifact is from a mirror image previously ruptured ophthalmic.

Another example below — same patient right and left injections. Left ICA is dual supply. The meningeal branch of the ILT is shown by arrowhead.

Anaglyph
Cross-eye stereo pair

Now, what’s going on with the strange tortuous course of the RIGHT ophthalmic? Well, well… another embryology idea here — that early in development there is a ring that forms around the optic nerve between primitive dorsal-like and ventral-like ophthalmics. Failure of ring formation leads to dual orbital supply. In this case, the peculiar morphology might be an example of another consequence in ring formation deficiency in this patient. More on that later… For now, some nice images of this rare disposition

Ophthalmic-Middle Meningeal Relationship
Guess what? Good job — its a spectrum! The whole thing about variants like meningolacrimal, meningo-ophthalmic, recurrent meningeal, — its all part of a continuum in balance between ophthalmic and middle meningeal territories. The same kind of analysis as we did for origins of ophthalmic artery above will apply here also. Below is a figure from our MMA publication, where this idea was first shown. Its a busy slide, we will dissect it below.

In a most common, “classic” scenario, the ophthalmic supplies the orbit, and MMA the meninges. However, these territories are in balance. Not infrequently, one may encroach on territory of another. The partial, and thus relatively common, orbital supply of MMA is known as the classic meningolactrimal variant. The reverse is partial ophthalmic supply of the convexity meninges, limited to anterior branch. More rare is “complete” MMA supply of orbit — the meningo-ophthalmic variant. Correspondingly rare is complete ophthalmic supply of convexity meninges – both anterior and posterior branches. This simple idea unites the two types of MMA /ophthalmic relationships.
Also important is to understand that none of these dispositions are discrete. The “meningolacrimal” is not just MMA supply of lacrimal gland — it can be also superior rectus, inferior orbit, etc. Or MMA can supply almost the entire orbit, except for central retinal / ciliary territories. Its all part of spectrum and balance. Below are detailed examples
Meningeal Supply of Orbit
The classic meningolactrimal variant is MMA supply of lacrimal territory. The “lacrimal branch” enters the orbit via its own foramen of Hyrtl.

Frontal views are key — the lacrimal branch projects over the lateral orbit

Another example below — note blush of the lacrimal gland (beige) in parenchymal phase. The sphenoid branch — a key MMA branch that runs along the sphenoid ridge, like a railroad, connecting the various ophthalmic variants, is shown by white arrow. Lacrimal branch is brown. Faint visualization of the more distal sphenoid branch, where it will connect, to your peril, with the more proximal ophthalmic artery, is shown in red.

ICA injection of same patient shows globe (ciliary) supply via classic ophthalmic. The artery is slightly smaller than in its full expression, owing to its less than complete orbital supply

Greater MMA supply of orbit
Again, the spectrum. The continuum is shown below, meningeal contribution to the orbit inclues both the classic “meningolacrimal” artery via foramen of Hyrtl (arrowheads) and superior orbital fissure supply (dashed arrows) that manly goes to the posterior ethmoidal foramen (ball arrow). Also present is a rare but important deep recurrent meningeal branch (arrow) supplying area of ILT and going as far back along the tentorial arcade as tentorial incisura…






Movie of DYNA axial data — pause and scroll thru images
Nearly complete MMA supply of orbit — the meningo-orbital variant.
Rarer, of course, since it represents a more complete takeover by MMA of the orbit — but not as rare as one might believe, since it is not discussed as frequently as the “meningolacrimal” variant. That’s another error we want to correct. In this kind of spectrum, we see MMA primarily supplying most of the orbit — except that there is no choroid blush. That comes from a gracile ophthalmic artery, which seems to be limited to supply of the nerve and ciliary vessels. Since it does not have a name, we hereby name it the meningo-orbital variant. Well, why not? Again, there is nothing new under the sun. In words of the great Hayreh (based on his observations and the literature he studied extensively): “It is very rare for the entire orbit, including the central retinal artery, to be supplied from the middle meningeal artery. The central artery of the retina usually preserves its connexion with the cerebral circulation by arising from the internal carotid even though other branches in the orbit may arise from other sources such as the middle meningeal“
In this example, courtesy Dr. Daniel Sahlein — ophthalmic supply limited to nerve and globe — notice also the relatively distal origin of the ophthalmic — the classic hypophyseal segment — see spectrum above 🙂

DSA of MMA injection

Amazing DYNA MMA images, best of what’s available in 2021. Lets see what happens 10 years from now — the original ophthalmic artery page was made 10 years ago — see how much things have improved…

Another example below — there are extensive images here, with DYNA movies and a wealth of information related to supply of nerve, orbit, globe, etc. Bottom line is that ophthalmic supply is mainly to nerve and globe, via the long and short ciliary arteries (more on that below). The CRA is beautifully seen. The MMA supplies nearly all of the rest of orbit — except for probably one short ciliary branch — see that part of choroid blush? Its all a spectrum…
ICA

ECA

MIP DYNA ICA

MIP DYNA ECA
Stereos ECA

Stereos ICA

Beautiful co-registered ICA and ECA injection DYNAs — there is dual supply to the optic nerve — proximally its from MMA (white arrow), more distally / CRA is from ophthalmic (red arrow)




Movie
Stereos co-registered ECA and ICA — without cross-eye its hard…

Finally, movies — these are the best to understand this. Pause and scroll thru individual frames — axial, sag, and cor all in here.
ICA
ECA
An entirely almost totally meningo-ophthalmic — but not quite
See, thats the spectrum approach. Its a continuum. Here is the smallest so far in our experience contribution from the ICA and within its own foramen. Arrows below


MMA — sphenoid ridge branch (arrows) to ophthalmic. Another portion of sphenoid ridge branch is dashed arrows — to the lateral MMA. Arteries extending along the optic nerve/sheath complex towards the canalicular segment (ball arrows) — likely anastomosing with the ICA origin diminuitive ophthalmic artery

Coronal oblique DYNA CT images — the ophthalmic artery — likely anastomosing with the calnalicular segment of the ophthalmic above.

Bonus question — what is wrong with the image below?

Complete Meningo-ophthalmic
Finally, the least common, and of course most classic — complete supply by MMA. Probably the most famous “dangerous anastomosis” — and rightly so. This one is not an anastomosis, and the now Toronto Western group that published this classic paper is well aware of it. But its a catchy name, and if it helps save some eyes, that’s great. Plus, we will get to more anastomoses later… The point is — proximal MMA embolization risks blindness, in addition to facial nerve injury via petrosal branch and cavernous / ILT /MHT balance and corresponding Meckel cave nerves — see MMA page for that.
In this example by Dr. Eytan Raz, there is no ICA origin ophthalmic at all

Frontal ECA — see how medially the vessel projects over the orbit — dead center. A good rule of thumb is that anything that projects over the orbit in frontal ECA views is either the occipital, or meningo-ophthalmic. It should not be too hard to tell them apart, and must be worked out. Here, the catheter is beyond occipital origin — in which case whatever projects over the orbit is going to be in the orbit.

Lateral views — there is an obvious choroid blush. Other vessels — anterior deep temporal, septal / turbinate, facial branches can project over the orbit in lateral views also. The frontal view is more useful, except for the choroid blush

ECA MIP — the globe is well-lit. Another amazing deep recurrent meningeal continues as free margin of tentorium branch (Bernasconi-Cassinari), reaching the falcotentorial junction, and heading up as artery of the inferior sagittal sinus. Now, that’s not just curiosity — it’s an important lesson — because the same path contrast took to get there can be traversed retrogradely by an overzealous liquid embolic injection — so that one can end up in the orbit from a falcotentorial injection. Be careful.


Movies — again, wealth of info here — stop and scroll
ECA
ICA — notice something very curious — there IS a tiny indeed vessel present in the canalicular segment / optic canal area. The balance / spectrum approach might suggest that a tiny ophthalmic artery from the ICA could be present still. Why not? Granted, this is a 20 second DYNA, at those might be veins — but unlikely. Also, if they are veins in the canal, there must be an artery there as well. The more we look the more we see…

Over the orbit may be in the orbit
Cant be over-emphasized. Any ECA injection frontal view artery projecting over the orbit can be in the orbit. makes sense. What is it — occipital or meningolacrimal/meningophthalmic as a rule. Here, the catheter is below occipital origin — unlike in the above example. Occipital is dashed arrows, MMA solid, ophthalmic ball arrow, and choroid blush arrowheads. Boxes are corresponding orthogonal views

Had enough? Of course not. We are not even halfway there yet…
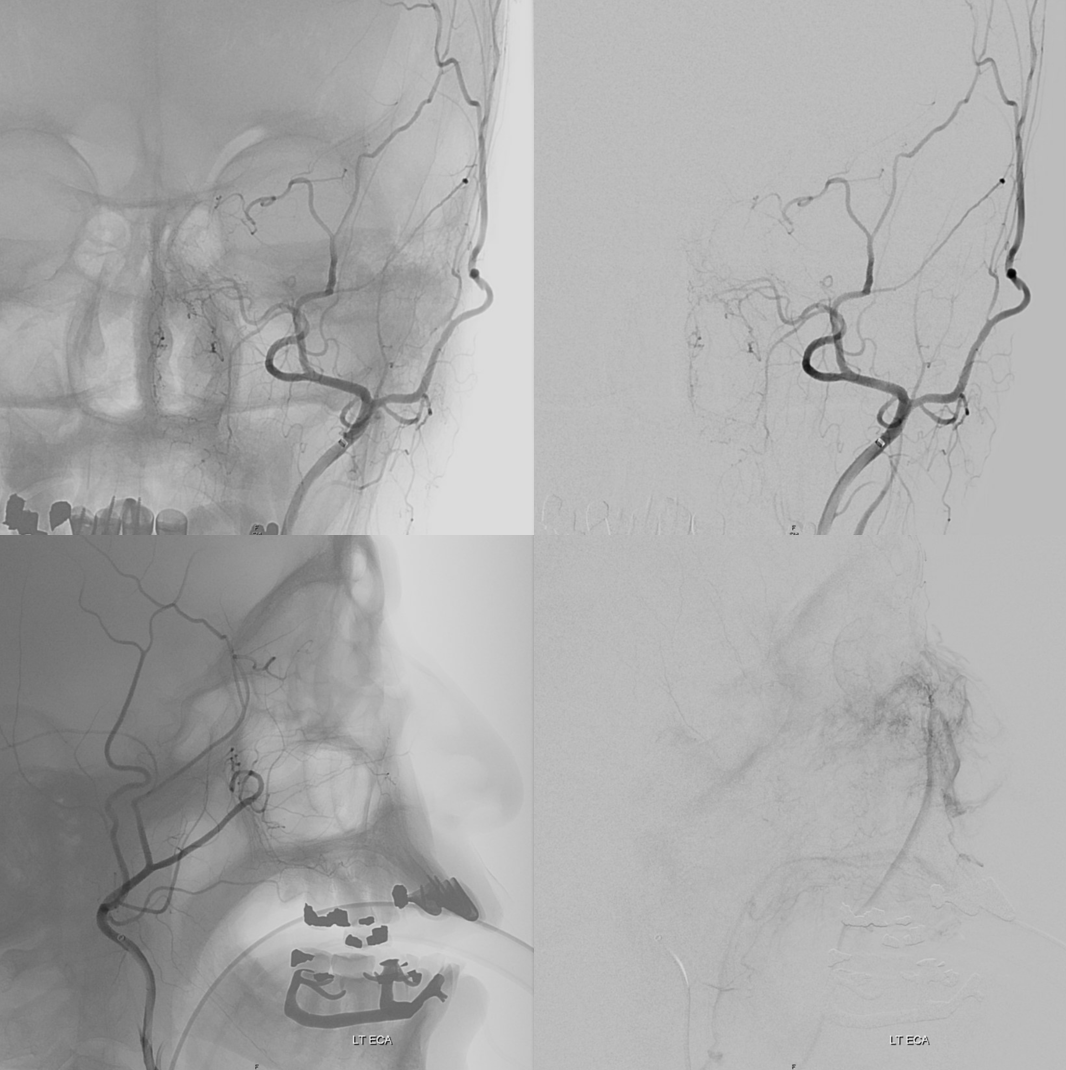
AccessoMeningOphthalmic
Now to the Super Rare. Accessory Meningeal Supply to the Ophthalmic. Why not? “Vessels are Everywhere” Dodi Boccardi. But, what’s the pathway? The accessory meningeal not infrequently contributes to meckel cave / cavernous region supply, in balance with the cavernous branch of the MMA and the anterolateral / meningeal branch of the ILT. Here, there is a dorsal ophthalmic — the anteromedial ILT branch.
For extra credit – what else is seen in the image on the left?

However, it seems like there is some kind of washout. Something else is also contributing.

The easiest way to figure this out is flat panel imaging. Below is a movie of the axial dataset of a 3D-DSA. Stop and scroll thru frames
See it — the accessory meningeal arises separately from the MMA — with the classic deep course of the IMAX. Once thru ovale, it heads medially towards Meckel cave — the cavernous branch region — joining the anteromedial branch of the ILT — a.k.a. dorsal ophthalmic. The picture is complete
Some stereos


MIP

This is the only example of such variant we know of. So there.
Recurrent Meningeal Artery Spectrum
The flip side to MMA takeover of ophthalmic territory is ophthalmic takeover of MMA — and spectrum again. At least 5% incidence. Complete takeover of MMA territory is rare (anterior and posterior MMA branch), partial (anterior branch only) is more common — makes sense from balance perspective. In the example below, recurrent meningeal (white arrows) supplies anterior branch only. It is best seen in the late arterial or parenchymal phase of ICA injection — meningeal territory resistance is more than brain, and flow is correspondingly slower in normal situations, compared with brain. Posterior branch (black arrows) is from ECA

Another older example, ICA injection only

Complete Recurrent Meningeal
See below

Frontal views

In this trauma case (full case here), skull fracture and associated meningeal fistula (red, very common with skull fractures, conservative management is usually fine) is supplied by recurrent meningeal artery (white). A few areas of contrast pooling from other fractures are yellow.



ECA shows no MMA over convexity

The clinical implication of this variant is that it may limit access to MMA territory, most commonly for MMA embolization, or trauma cases like the one above. Strategies can be pursued to indirectly embolize this territory without catheterizing the ophthalmic artery.
Hemodynamic Balance MMA/Ophthalmic/ECA
So far, we have treated these variants as part of a spectrum, but still quite static. The reality is more complex, with all kinds of hemodynamic balances between various territories. The connections between MMA and ophthalmic are present as ALWAYS as always can ever be in medicine. This was seen in ALL specimens analyzed by Hayreh and extensively verified angiographically by us.
These connections can be augmented of course — occlusion of cervical carotid for example will reverse flow in the ophthalmic in most people, as extensive external anastomoses with the ophthalmic will help support ICA territory. The typical examples of this are common. Here are some very old and still excellent examples

Sphenoid branch = green; Cavernous branch of MMA = red; ILT = brown; MMA = yellow. Ophthalmic = blue

Below is a very uncommon route — in a patient with occlusive ICA dissection — see reconstitution of ophthalmic in right image

Now look at this — reconstitution of the left ICA is also provided by RIGHT MMA (white arrows) to left MMA (meningo-ophthalmic, black arrows; sphenoidal branch -purple arrow) to ophthalmic artery (red arrow)! Notice also anterior meningeal branch (yellow) reconstituting ophthalmic via the ethmoid artery (pink arrow). Case by Dr. Eytan Raz

Flow diversion of paraophthalmic aneurysms is safe and effective because of ophthalmic-ECA anastomoses. The ophthalmic needs to close in many cases to allow for the aneurysm to close also, and this happens about 25% of time, overwhelmingly with no issues — see case below — ophthalmic (A,C — black arrowhead) has shut down asymptomatically in D, together with the giant aneurysm. Choroidal patency is maintained despite multi-device coverage — because, unlike the ophthalmic, it has poor collateral support.

The MMA-ophthalmic relationship is part of this network — there are numerous anastomoses present, that can be uncovered by injection technique or flow constraint. Angiography is the best way to understand these dynamic connections. Obviously they are very important. Just because one may not see an anastomosis between MMA and ophthalmic does not mean its not there. Below are some examples:
ICA injection shows typical ophthalmic (red)

ECA injection — notice there is spasm around the catheter — which changes the hemodynamics — reducing antegrade flow in the ECA, while allowing for more perfusion pressure during injection — and demonstrates MMA/ophthalmic anastomoses, retrogradely filling the ophthalmic (red), with choroid blush (purple)

Frontal views, as often, are more revealing. The sphenoid branch in early phase of injection is white. In later phase (lower left image), with spasm below, there is transient opacification of the ophthalmic artery (red) and the ethmoid branch (pink). The still later phase (lower right image) shows lacrimal gland blush (yellow)

Enough? But we haven’t even gotten to the main part yet… So far, we’ve just been talking about origins and connections. What about the opthalmic artery itself? Lets GO!!!
Ophthalmic Artery Course and Intraorbital Vasculature

The recent tremendous advances in flat panel CT let us achieve astounding things. Each dataset is like a whole world of anatomy. The beautiful ex-vivo images of casts are now possible in vivo, with resolutions well below 50 microns — truly amazing. Most of this pioneering work, especially in-vivo visualization of central retinal artery, is led by Dr. Eytan Raz. Not since the lifetime of work by Sohan Singh Hayreh (refs 1 2 3 for example — are a MUST read for anyone interested in ophthalmic artery — together with Surgical Neuroangiography there are no better literature references) has there been an advance in visualization of ophthalmic artery like this. We are truly at the forefront here, but hope that others will pick it up and look as well — its amazing what you will find.
Below is a page from another anatomy genius — the great Frank Netter. His gift for artistic depiction of anatomy — no computers, no injections — just eyes, paper, paint, and genius (!) — remains unsurpassed by anyone known to me over the history of civilization, in my opinion. If you are a medical or another anatomy student, and your school is not using Netter for some reason, just get it yourself (mine had us buy Netter and another atlas made by our anatomy instructor 🙂 — we were used to this kind of treatment since college). Basically, all of the info below is summarized in these Netter image

As we mentioned at the beginning, the orbit is a unique structure, in that it it houses both face and brain. The optic nerve and globe are an extension of the brain, with typical poor collateral vascular support. Certainly poor compared with the rest of orbit, which is just like face — muscle, fat, glands — the collaterals to those are practically inexhaustible. Naturally, we are preoccupied with supply to nerves and globe, but other stuff needs to be discussed also.
General Ophthalmic Artery Anatomy (typical ICA origin)
There are 3 segments, from external landmark perspective — intracranial, intracanalicular, and intraorbital. The first two we basically covered from an angiographic perspective.
It is important to appreciate that the study of anatomy is a way of seeing — an art — and the methods of seeing vary. Our method is angiographic and hemodynamic. There is a wealth of literature on anatomical relationships between the ophthalmic artery, the optic nerve, and adjacent dural /osseous structures. The knowledge of these relationships continues to be important for some specific surgical applications — such as meningioma surgeries. However, endovascular methods are now the mainstay of paraophthalmic aneurysm treatments, where external landmarks are of minimal importance. The main question is whether an aneurysm is intra- or extradural, and the dural rings cannot be seen by any modern imaging modality yet — ICA page for more on that. Most ophthalmic artery origins beyond the anterior genu are considered intradural. For the interested reader, the treasure that is the Rhoton Collection (free with creation of a login and password), Hayreh references 1 and 2, and the Neurosurgical Atlas Website are recommended.
The first two segments of ophthalmic artery — intracranial and intracanalicular — usually have no branches that we can see, even with DYNA most of the time. The intraorbital segment is of course the largest one. You can break it down into simple functional parts. There are the central retinal artery and short and long ciliary arteries supplying the globe. There are muscular arteries to all muscles. There are branches to the lacrimal gland. There is a supraorbital artery that typically reaches up to the eyelids (eyelid arcades) and skin of the brow and forehead. Finally, there are the anterior and posterior ethmoid branches — which can supply the anterior meningeal artery and a variable amount of nasal / septal tissues. There is balance in and with everything — facial, middle meningeal, etc.
The overall diameter of a dominant ophthalmic artery of course varies, depending on amount territory supplied and balance with other sources, as above. Generally it is 1 mm or slightly larger — certainly big enough for a 17-size microcatheter. Our current favorites for this job are the single tip 167 cm, 1.3 F (0.45 mm OD) Headway Duo or the 1.2F and 1.5F Magics.
Ophthalmic Artery Ring
The intraorbital course of the ophthalmic artery in relation to the optic nerve can be understood in terms of the embryologic ophthalmic artery ring concept, as explained in the classical Surgical Neuroangiography. The idea is that an anastomotic ring forms around the optic nerve between early vascular contributors to orbital supply. A bit later, the ring breaks, with adult course of the vessel(s) determined by the part of the ring that stayed (including aforementioned MMA contributions). The image below — from Surgical Neuroangiography — illustrates the ring formation (E) and breakup (F) concepts

Just like the overall ophthalmic artery can be subdivided into 3 segments (intracranial, intracanalicular, and intraorbital), so can the third, intraorbital segment, be further subdivided into another 3 parts (see Hayreh) — proximal (1), angle (2, ring area), and distal (3).
The intracanalicular and proximal intraorbital (part 1) ophthalmic artery pretty much always courses below, or below and lateral to optic nerve, whereas the distal intraorbital part (3) courses medially and above it. Usually (83% in Hayreh), the lower part of the ring regresses, which results in the ophthalmic crossing over the optic nerve (using the persisting upper part of the ring), and giving the vessel its classic “bayonet” appearance — from the days when doctors did not mind naming anatomical concepts after instruments of death. In the remaining 17%, the opposite happens (upper part of ring regresses, and ophthalmic artery crosses under the optic nerve). This results in a straighter course of the artery. The image borrowed by us from Hayreh (red notations added by us) shows the idea.
Below is an old image of this, from early thrombectomy days. The key thing about the “bayonet” is that the central retinal artery (one of the yellow branches — can’t tell which one — most are ciliary and only one is retinal — see more below) nearly always arises from the second portion of the vessel (the angle) — or the first bend of the vessel. Other vessels here are: blue = nasal septal via anterior ethmoids; bright green and pink = supraorbital and supratrochlear; purple = upper eyelid; light green = lower eyelid; black = lacrimal

How did we ever figure out all those branches on an analog machine, without functional rotational capabilities? Stereo imaging. Check it out here. Cross-eye stereo images below

Old schematic of relationship to optic nerve

Want to have it easy — sure — here is a DYNA — of a patient post supraclinoid carotid artery stenting in setting of acute ischemia — see full case here. Coronal views (stop and scroll) show artery crossing over the nerve — the central retinal can be traced here (as in every DYNA).
As in anything, there is a spectrum. The ring, and hence the bend in the opthalmic where it crosses under the optic nerve, can vary in position, sometimes close to apex of orbit (as it is above), sometimes quite far. In the example below, the under-the-optic-nerve ophthalmic segment is very long, and the angle (part where it crosses over the nerve) is just behind the globe. Nevertheless, the central retinal artery again takes off right where the bend its. This of course is key information when embolization via ophthalmic is needed.

The same rule of the bend and CRA usually holds true for the above-described spectra of MMA/ophthalmic co-existence in orbital supply — see image below that was shown above also

Below is the example of 17% where the opposite happens, and the course of the artery is a more gentle, if you will, upswinging curve crossing under the optic nerve– see older image of this below. Also seen in this image are well-developed supraorbital artery (purple) going to the skin of the forehead, and anterior and posterior ethmoid systems supplying much of the nasal septum (pink) and skin of the nose (anterior nasal artery, white)
A more modern example of a dedicated ophthalmic artery injection — same less angulated course


Movie — this is actually a very interesting case of what is probably ciliary artery spasm — notice how we can see here isolated retinal blush — not choroidal! The patient had visual decline following pharmacologic stress testing — probably inducing some sort of orbital vasoconstriction. Speculative, but very interesting — and good images.
This, by the way, is the same case we showed way above — with the two unusual things about the image (remember?). The one unusual thing is ophthalmic artery coursing under the optic nerve. The other is lack of choroidal blush in this phase of injection

The occasional persistence of dual orbital supply that we showed above — say by both classic and dorsal ophthalmic arteries (Lasjaunias way) — is thought to result from failure of some kind of failure in ring formation. It is useful to know this unproven but important idea.
Please also pay attention to the relationship between ophthalmic artery and optic nerve in the many DYNA flat panel movies below
Intraorbital Ophthalmic Artery Vasculature
Can be divided, for purposes of safety and importance, into Central Retinal Artery (CRA), the short and long Ciliary Arteries, and everything else.
The amount of variation in order of origins and size of vessels is tremendous. The basic guide that holds true more often than not is again discussed in Hayreh: branching pattern is most influenced by whether the artery crosses over or under the nerve. Makes sense. Below is again a borrowed image from Hayreh, showing the differences. What is nearly always the same, and key, is that the central retinal and ciliary arteries will be originating somewhere along the angle (bend) in the vessel.

The basic idea is that in the majority of over-the-nerve cases the central retinal will come off more proximally around the bend, whereas for the uncommon under-the-nerve cases it will originate a bit more distally (but still where the bend is) — the medial posterior ciliary branch in this case often coming off first. We want to re-emphasize that these are minor differences in the big scheme of intra-orbital embolizations. The key part when working in the orbit is to understand the simple idea that the more distal from the bend/angle, the better.
Central Retinal Artery

About 200-250 microns in diameter (relevant for particle embo). DOES have some collaterals with ciliary arteries, but generally insufficient in cases of acute occlusion as far as we know — especially for central vision. Usually arises where ophthalmic artery swings either below or above the nerve — see above. Very difficult to see on angio — especially to distinguish from cililary arteries — and 100% consistently seen on DYNA — as pioneered by Dr. Eytan Raz. For practical purposes, staying well distal to the ophthalmic artery bend is best if embolization in ophthalmic territory is contemplated — see examples of cases here.
Orbital Metastasis Pre-Operative Embolization
Ethmoid Fistula Ophthalmic Artery Route Embolization
Orbital AVM Preoperative Embolization — particularly important case to highlight anatomy, various ophthalmic-ECA anastomoses, nBCA technique, etc.
The choroid blush can be present on angio with CRA occlusion, since the bulk of this blush is made by ciliary arteries, and not the CRA. In other words, having a choroid blush does not mean much — one can be blind with it and see without it — but it is psychologically relevant to the operator.
In the case below, there is extensive nBCA cast (right image) in the meningo-ophthalmic artery (pre-nBCA image on left — arrowheads on both) after a disastrous glue injection

Post ICA and ECA injections show neither choroid blush not any evidence of ophthalmic artery at all — granted the phase is early, maybe something would show up in venous phase

The patient woke up with perfect vision — and stayed that way. This is certainly due to overwhelming good fortune — and a major cautionary tale — but it shows that one can have vision without at least 2-D angiographic evidence of choroid blush
Below is a typical lateral stereo of ophthalmic artery injection. The choroid blush is well-seen. It is impossible to say however which is the CRA

DYNA thin MIP view of the same injection with excellent view of CRA — note typical over the optic nerve angle of the artery
a thinner MIP shows the optic cup

Optic Disk can usually be appreciated in coronal views. This is the limit of current resolution — of course vastly inferior to fundoscopy. But, for anything posterior to the globe — DYNA is by far the best we have

A duplicated central retinal artery (arrows below) converging on single vessel in this case is not so rare — again the more you look the more you see.

A large ciliary artery is present in this patient also — in this case arising more proximally than the CRA — also see DSA images of the same case above

Below is a movie of axial, coronal, and sagittal DYNA images of this patient — pause it and scroll thru individual frames.
Circle of Zinn-Haller — yes, we can see that also…

Axials

Movie — axial
Movie — coronal oblique perpendicular to optic nerve — notice faint vessels supplying the more proximal portion of the optic nerve — canalicular and orbital apex segments, as well as the Zinn-Haller…
Timing is everything
There is a central retinal vein also. DYNA/CONE Beam CT often averages arteries and veins. Telling apart artery from vein is not so easy sometimes.
Patient presents with sudden onset vision loss. Angio is classic — globe still there — because of choroid blush — choroid supplied by long and short ciliary arteries. Presence of blush does not rule in our rule out blindness

DYNA CTs — see the difference between 5 and 8 second acquisitions — there is a 1 second delay with 4 and 7 second “DYNA” options currently available on the Siemens Icono.


Proximal Optic Nerve / Chiasm/Tract
The central retinal artery is relatively small, by macroscopic standards. Yet, most of its volume goes to the retina. The nerve itself has truly microscopic requirements. Which is why, proximal to the CRA, there is usually no “central” vessel or angiographically obvious supply without a microscope. But, with flat panel imaging, we are getting there. The vessels there will supply both the optic nerve sheath and nerve itself. Visualization is inconsistent at present time. However, as in everything, it is clear that there are variations. In some cases, like the one below, visualization is particularly stunning. In addition to CRA (arrow), there are vessels surrounding the canalicular (arrowhead) and intracranial (dashed arrows) portions of the optic nerve and, amazingly, the optic tract (ball arrows). Its a 20 second DYNA, so some of them are are veins for sure.

Magnified view

Coronal views are excellent to show that those proximal nerve vessels are not in the nerve, like CRA, but supply the nerve from the surface — makes sense, like all other nerves

Coronal movie of the same
Look at those retinal branches!

Movie
Coronal MIP — super visualization for flat panel 2021

Vorticose Veins and Optic Papilla
Case Study — A single case insight into many aspects — each injection is an anatomical cornucopia… click here for more
Optic Nerve / Pituitary Supply! — ischemic disease
DYNA offers a whole world of insight — no one suspected it before. Amazing stuff. Look at this acute stroke code — critical petrous segment stenosis, perfusional exam — straightforward stenting. Note that ophthalmic is missing before and after stents

ECA run — typical meningo-ophthalmic right? Not much else to see
Well, check out these DYNA MIPs — look at proximal optic nerve / chiasm/ optic tract supply! and ACA / ventral ophthalmic channel also… Movie (pause and scroll) shows MMA entry into orbit via foramen of Hyrtl. The embryologic implication of this is that MMA supply to orbit in this case is acquired rather than developmental — in latter cases (complete meningophthalmic variant) the MMA entry into orbit is via the SOF. Probably, supply of optic tract/chiasm and pituitary (incredible) is an adaptation to petrous stenosis hypoperfusion also. Incredible…

High res movie — ICONO machine
Ischemic Optic Neuropathy — note small irregular caliber of the CRA
Central Retinal Artery Occlusion (CRAO)
Case Courtesy Dr. Erez Nossek. The classic finding of preserved choroidal blush on both DSA and DYNA CT — however the patient has a clinical CRAO and the retinal artery is absent on the DYNA. What you see is the enhancement/blush of the choroid, not the retina



In this patient the majority of orbital supply comes from the MMA — however this is not a factor in the CRAO. The MMA does not supply either the retina or the choroid — but everything else in orbit yes. See the “spectrum” concept above

Ciliary Arteries
Example of Isolated Ciliary Artery injection — there is no central retinal artery here. It looks very much like CRA on 2D-DSA. The difference on DYNA is taht we do not see the papilla or the characteristic straight course towards it. The ciliary artery is much larger than the CRA, and tortuous just postrior to the globe — so as to allow for movement of the eyeball. Note presene of vortical veins draining into superior and inferior ophthalmic veins. One of the most beautiful images on neuroangio.org
Contrast this with injection below — where both CRA and ciliary artery are seen. See the difference? BTW, the straight vessel coursing posterior to the CRA along the optic nerve is the central retinal vein, which often extends much more posteriorly than the site of CRA entry into the sheath / nerve.
GIFS — for reference


2D – DSA ciliary artery


Labels. CRA = arrow; ciliary = dashed arrow; central retinal vein — arrowheads

DYNA coronal MIP

Labels. CRA = white arrow; Ciliary = dashed arrows; central retinal vein = black arrows. Notice delineation of the optic nerve within the sheath. This is actually not normal — reflecting atrophy / Wallerian degeneration

Ethmoidal Arteries
Of course, vessels don’t read books or websites. Another example here — there are 3 ethmoidal sets of arteries — posterior (dashed arrow), anterior (arrow), and whatever else we want to call them — lets say anterior-most (arrowhead)

Movie of the same subject below — this is a case of penetrating sharp object orbital trauma — notice major swelling of the anterior orbit / eyelids, and an irregular “guitar pick” shape of the globe due to increased intraorbital pressure, which is maintained in perfusion range by prior canthotomy. The extra-ocular muscle vessels are particularly well-seen, and their anterior ciliary tributaries to the anterior chamber, thanks to post-traumatic hyperemia.
Same subject DYNA MIPs. Anterior ciliary arteries are labeled

Ethmoidal Dural Fistula
Arterial supply to these will be from whatever goes to the cribriform plate — which means ethmoid arteries are usually involved. The anterior meningeal artery is also — and usually in retrograde fashion — flow is reversed into the fistula. Other supplies can be from IMAX via reversal of flow in the septal system. Anterior ethmoid artery embolization is increasingly used as a route. The anterior ethmoid is quite distal from the CRA. When liquid embolics are used, risks include reflux of embolic, arterial injury during catheterization, or injury during the “pull”. For examples of various techniques — see Ophthalmic Artery Embolization of Ethmoidal Fistula, Anterior Meningeal Route Embolization, and Retrograde Transvenous Embolization
Supply of Anterior Meningeal Artery
See Meningeal Arteries page for that. Its important. This meningeal network is key. The above-mentioned case of Anterior Meningeal Route Embolization is another great example of this kind of connectivity. It is also a potential danger when liquid embolics are used for MMA embolization, as the liquid can travel a great distance along the anterior meningeal artery so we are very careful to watch that area.
The diagram below gives the idea

A well-seen anterior meningeal network shown below — even bifurcating (white arrows) with both arteries in wall of sagittal sinus. There is a meningeal blush (black arrow). The size of anterior meningeal artery no doubt is enlarged to compensate for loss of the MMA post left pterional craniotomy and clipping of a ruptured PCOM. Angio is done for spasm Rx.


Ethmoid Autosynangiosis in Moya Moya
Ethmoid arteries participate in dural supply — as evidenced in their supply of cribriform plate meningiomas for example, or the aforementioned dural fistulas. Another example is an autosynangiosis, typically with the anterior frontal / gyrus rectus branches of the anterior cerebral artery in cases of Moya-Moya. In example below, courtesy Hima Pendharkar, the supply mostly comes from anterior ethmoid group — notice however that there are two branches present, both anterior — again, they don’t read books.

The other side. Anterior ethmoid group is marked by arrows. The posterior group also participates, and is unmarked.

Some amazing DYNA imaging by Dr. Eytan Raz — anterior ethmoidal = yellow; recipient frontopolar/ rectus branches of ACA = green; posterior ethmoid branches supplying the nasal septum = blue; anterior meningeal artery = white’ supraorbital/supratrochlear branches = black


Movie
Stereo angios of another example

Supraorbital Branch
Typically the highest branch in the orbit, supplies whatever is near it (superior rectus, oblique) and comes out of orbit, where its in balance with the facial and temporal arteries. Can supply a lot of skin over the brow as supraorbital/supratrochlear arteries, skin of nose as anterior nasal artery. Not to be confused with the anterior meningeal artery that is usually supplied by anterior ethmoid branch — the latter is inside the skull and midline, whereas supraorbital is on surface and off midline
Stereo views below


As mentioned above once or twice, everything is in balance. Here, prominent supraorbital branch of the OA supplies skin of the nose. The infraorbital artery from the IMAX supplies the angular region. There is corresponding hypoplasia of the facial and transverse facial arteries

Stereos

Anaglyph
Old example –stereo pair — supraorbital branch is purple. Nasal is white
Re-showing image from above — supraorbital (pink) and supratrochlear (bright green) cutaneous vessels.

Lacrimal Branch
Covered above mostly. When MMA participates in orbital supply, that’s the most common part it grabs. The laterally–projecting branch to the lacrimal gland (cream-colored arrows below) in the meningolacrimal example below. Foramen of Hyrtl, etc. etc.

Usually, however, lacrimal branch is more supplied by ophthalmic artery. Since it is located in the lateral orbit, there will be frequent anastomoses with the anterior deep temporal branch of the IMAX or the zygomatic branch of the STA — good routes for collateralization of the ophthalmic system and intradural ICA when proximal ICA is occluded. See these older but still MOSTLY true neuroangio.org images


Below is an in-vivo example:
Multiple routes of ophthalmic artery reconstitution: superficial temporal via supraorbital artery (arrows), MMA (dashed arrows), infraorbital (open arrows), and facial/angular (ball arrows)

Especially well seen are superficial temporal and angular / nasal anastomoses.


Old image of anterior deep temporal reconstitution. There is also reflux into the vert with a major PCOM reconstitution source

Of course — to end as how we began — everything is balance/spectrum. In this case of ICA injection, the frontal territory of superficial temporal artery (dashed arrow) is supplied by the lacrimal branch (arrowhead) of the ophthalmic — typical small foramen in lateral orbital wall (arrow)

ICA reconstitution via Ophthalmic Artery
The most efficient by far ECA to ICA cervical carotid reconstitution is via the Ophthalmic Artery. Tons of possibilities — via MHT, anterior deep temporal, facial, ethmoid/anterior falcine, etc. Here, in case of proximal right ICA dissection, there is a really nicely seen STA to supraorbital anastomosis, and an anterior deep temporal to lacrimal branch also.

Can you see a myriad others on this MIP image?
Ophthalmic VEINS
Well, veins are not arteries. But we still want to show a bit here. They are best seen on a common carotid injection — since supply is usually from ICA (classic ophthalmic) while venous drainage is often from ECA (SOV and IOV towards cavernous sinus). In cases of increased intracranial pressure, or very occasionally without it, the drainage is retrograde and better seen from ICA injection alone. Here is one such case — pure venous phase
Stereo


Applications
Ophthalmic Artery Menigioma Embolization
Ophthalmic Artery Embolization of Ethmoidal Fistula
Intraorbital Metastasis Embolization
Ischemic Optic Neuropathy Case
Good stuff? This is no book — we are updating constantly… Feedback goes to [email protected]


