Photoessay of the cutaneous manifestations of the idiopathic inflammatory myopathies
Published Web Location
https://doi.org/10.5070/D31f04d17zMain Content
Photoessay of the cutaneous manifestations of the idiopathic inflammatory myopathies
Elizabeth M Dugan1, Adam M Huber2, Frederick W Miller3, Lisa G Rider3, for the International Myositis Assessment and Clinical Studies (IMACS) Group4
Dermatology Online Journal 15 (2): 1
1. Division of Dermatology, Department of Medicine, Georgetown University Hospital, Washington, DC2. IWK Health Centre and Dalhousie University, Halifax, Nova Scotia, Canada
3. Environmental Autoimmunity Group, National Institute of Environmental Health Sciences, National Institutes of Health, DHHS, Bethesda, Maryland. [email protected]
4. Contributors to this photoessay listed in the appendix.
Abstract
Adult and juvenile dermatomyositis, polymyositis and myositis overlapping with another connective tissue disease are rare systemic autoimmune diseases with a primary feature of weakness and muscle inflammation. Cutaneous findings specific to the underlying condition are present in many patients with these disorders. Some lesions are highly characteristic of the idiopathic inflammatory myopathies (IIM), especially in dermatomyositis. Some cutaneous findings are common but not specific to the IIM and others are less frequently observed in patients with these illnesses. Many of these manifestations also have different grades of disease activity or damage. This photoessay reviews the classification and assessment of the cutaneous manifestations of the IIM and presents example photographs of many of the lesions of adult and juvenile IIM accumulated from the clinical experience of international experts in these conditions. The purpose of this work is to facilitate better recognition of the diverse cutaneous manifestations associated with these inflammatory myopathies.
See accompanying review article Review of the classification and assessment of the cutaneous manifestations of the idiopathic inflammatory myopathies
Explanation of the Slides
Please Note: As with all of the Dermatology Online Journal's articles, click on each image for a full-size enlargement.
Introduction
The following slides are examples of cutaneous manifestations seen in patients with the idiopathic inflammatory myopathies, including adult and juvenile dermatomyositis, polymyositis, and myositis associated with other connective tissue diseases. The framework for this photo-essay is modified from the Cutaneous Assessment Tool for the assessment of myositis, which is designed to catalogue and grade the severity of the cutaneous findings described in the adult and juvenile idiopathic inflammatory myopathies [1]. This photo atlas is meant to be used with the Cutaneous Assessment Tool, but can also be used as a teaching tool independent of the Cutaneous Assessment Tool. Both active and chronic persistent or irreversible lesions are included. Activity refers to the presence of underlying inflammation or vasculopathy [2]. Active lesions include those that are characteristic (Gottron's papules and heliotrope rash), erythematous lesions, vasculopathic lesions, and hand changes. Damage constitutes lesions that persist for at least 6 months that result from prior active disease or therapy [2]. Changes viewed as damage are often post-inflammatory or irreversible. This photo-essay also includes representation of these cutaneous manifestations of the IIM in patients with a variety of baseline pigmentation skin types; the subtleties of these lesions can be overlooked or misinterpreted in patients with darker skin types (types III-VI). In addition, cutaneous manifestations that result from symptomatic pruritus are also included because this is an under recognized symptom of dermatomyositis [3] and may help to distinguish dermatomyositis from the cutaneous lesions of lupus erythematosus in which pruritus is not a feature.
I. Characteristic Lesions of Dermatomyositis
I. A. Gottron's papules
This is considered a hallmark sign of dermatomyositis. Primary lesions consist of erythematous to violaceous papules and plaques over the extensor surfaces of metacarpal and interphalangeal joints and over other large joints, including knees, elbows, and ankles, generally in a symmetric distribution. Secondary changes can be present, including scale, crust, erosions and ulceration in active lesions, or dyspigmentation (hypo/hyper-pigmentation), telangiectasia, and atrophy in more chronic stages or at resolution. In severe disease, ulceration and/or necrosis may occur.
 |  |
| Figure 1 | Figure 2 |
|---|
Figure 1. Gottron's papules. Discrete erythematous papules overlying the metacarpal and interphalangeal joints in a patient with juvenile dermatomyositis. (Click image for enlargement.)
Figure 2. Gottron's papules. Erythematous to violaceous raised papules overlying the metacarpal and interphalangeal joints in a patient with juvenile dermatomyositis. (Click image for enlargement.)
 |
| Figure 3 |
|---|
Figure 3. Gottron's papules. Characteristic raised erythematous papule overlying the proximal interphalangeal joint in a patient with dermatomyositis. (Click image for enlargement.)
 |  |
| Figure 4 | Figure 5 |
|---|
Figure 4 & Figure 5. Gottron's papules. Erythematous plaques overlying the elbows in two patients with juvenile dermatomyositis. In some patients, small erythematous plaques may overly the extensor aspects of larger joints, such as the elbows, knees, and medial malleoli. This is considered to be an extended part of the spectrum of Gottron's papules. Note in Figure 5, a focal area of dystrophic calcification at the site of Gottron's papules, which is indicative of damage, as discussed below. (Click image for enlargement.)
 |  |
| Figure 6 | Figure 7 |
|---|
Figure 6. Gottron's papules with ulceration. (Click image for enlargement.)
Figure 7. Gottron's papules with necrosis. (Click image for enlargement.)
The changes illustrated in the following images are indicative of damage at the site of Gottron's papules. These include atrophy, telangiectasias, calcinosis or dyspigmentation in a distribution of Gottron's papules.
 |  |
| Figure 8 | Figure 9 |
|---|
Figure 8. Gottron's papules with atrophy. Thinning and flattening of the epidermis and dermis in a previously active Gottron's papule.
Figure 9. Gottron's papules with telangiectasia. Gottron's papules with prominent atrophy, porcelain white scarring, and telangiectasia.
 |
| Figure 10 |
|---|
Figure 10. Gottron's papules showing secondary atrophy and telangiectasia in a girl with severe juvenile dermatomyositis. These changes are seen in association with prominent periungual erythema, a cutaneous finding indicative of ongoing disease activity.
Gottron's papules with dyspigmentation (hypo- or hyperpigmentation). Hyperpigmentation may mask underlying erythema in patients with darker skin types, and therefore may be indicative of ongoing activity, or it may represent inactive or chronic change. It is important to palpate hyperpigmented lesions to determine if there is underlying blanchable erythema.
 |
| Figure 11 |
|---|
Figure 11. Gottron's papules with hypo- and hyper-pigmentation. This image demonstrates central porcelain white hypopigmentation over the proximal interphalangeal joints, indicative of damage, with surrounding hyperpigmentation, in a distribution of Gottron's papules.
I. B. Gottron's sign
Symmetrically distributed erythematous to violaceous macules or patches in the same distribution as Gottron's papules. Characteristic of dermatomyositis, but not pathognomonic, because it can also occur in other conditions, although much less frequently.
 |  |
| Figure 12 | Figure 13 |
|---|
Figure 12. Gottron's sign. Confluent macular erythema with scale confined to the skin overlying the patellae in a girl with juvenile dermatomyositis.
Figure 13. Gottron's sign involving the skin over the patellae in association with linear extensor erythema (see definition II. A. ii) over the thighs, resolving as hyperpigmentation and silvery scale in an African-American woman with dermatomyositis.
 |  |
| Figure 14 | Figure 15 |
|---|
Figure 14. Gottron's sign, with confluent macular erythema, lichenification (accentuation of the normal skin lines secondary to rubbing), and scale over the extensor surfaces of the metacarpal phalangeal joints.
Figure 15. Gottron's sign. This image illustrates Gottron's sign with secondary erosions and crusting.
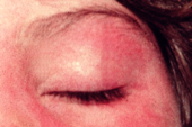
I. C. Heliotrope
A hallmark (i.e., pathognomonic) sign of dermatomyositis, consisting of violaceous to erythematous discrete or confluent macules confined to the upper eyelids. An eruption with similar characteristics can extend periorbitally. Heliotrope is often associated with periorbital edema and telangiectasias of the upper eyelids. In the resolution stage, atrophy or dyspigmentation (hypo- or hyperpigmentation) may be apparent.
 |  |
| Figure 16 | Figure 17 |
|---|
Figure 16. Heliotrope. Confluent macular erythema confined to the upper eyelid, with associated periorbital edema.
Figure 17. Heliotrope. Subtle erythema and minimal edema involving both upper eyelids, with extension to the lower eyelids.
 |  |
| Figure 18 | Figure 19 |
|---|
 |
| Figure 20 |
|---|
Figures 18, 19 & 20. Heliotrope. In patients who have darker skin types (type III - VI skin), heliotrope can be subtle and perceived as inactive or normal, resulting in under-diagnosis in this presenting sign of dermatomyositis.
 |  |
| Figure 21 | Figure 22 |
|---|
Figure 21. Heliotrope, resolving as telangiectasia: In this patient, heliotrope previously involved the upper eyelids; presently, only telangiectasias and atrophy are apparent.
Figure 22. Poikilodermatous change in a heliotrope distribution in a patient with chronic skin manifestations of juvenile dermatomyositis.
II. Erythematous Lesions
Includes sun-exposed, non sun-exposed and erythrodermic lesions, either as macular erythema or with accompanying secondary changes, such as scale, crust, or erosion. May vary in intensity from pink to red to violaceous. In patients with darker skin types (type III to VI skin), shades of "erythema" are more difficult to detect and clinical signs of activity may include blanchable erythema and increased warmth.
II. A. Erythematous lesions, sun-exposed
Includes malar and facial erythema, linear extensor erythema, V-sign, Shawl sign, and other photodistributed rashes. These erythematous eruptions are present in dermatomyositis, but generally not seen in polymyositis.
II. A. i. Malar and facial erythema
Confluent erythema in a malar distribution (involving the cheeks and extending over the nasal bridge) or more extensive involvement of the face including perioral, forehead, lateral face and ears. With malar erythema, nasolabial folds are often spared in dermatomyositis, which differs from systemic lupus erythematosus.
 |  |
| Figure 23 | Figure 24 |
|---|
Figure 23. Malar and facial erythema. Acute onset of confluent macular erythema in a periorbital and malar distribution with extension to the chin in a girl with juvenile dermatomyositis. Note the perioral sparing.
Figure 24. Malar and facial erythema. Striking malar and facial erythema with facial edema and scale represents a recent disease flare in an adult patient with dermatomyositis.
 |
| Figure 25 |
|---|
Figure 25. Facial erythema. Erythema and prominent hyperpigmentation involving the face and upper chest (V-sign) in an African American patient with skin type III - IV.
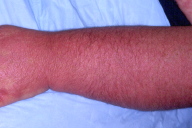
II. A. ii. Linear extensor erythema
Symmetric confluent macular erythema of the skin overlying the extensor tendon sheaths of the hands, forearms, thighs and/or feet in a patient with dermatomyositis.
 |  |
| Figure 26 | Figure 27 |
|---|
Figure 26. Linear extensor erythema. Confluent erythema of the skin overlying the interphalangeal and extensor tendons of the hand, with extension to the forearm in a patient with dermatomyositis. Note the association with Gottron's sign. This is distinct from cutaneous lupus erythematosus, in which there is absence of erythema over the metacarpal and inter-phalangeal joints.
Figure 27. Linear extensor erythema involving the forearm. This image demonstrates linear violaceous discrete and confluent macules, and erosions secondary to excoriation. Pruritus is an under-recognized symptom of active dermatomyositis [3].
 |  |
| Figure 28 | Figure 29 |
|---|
Figure 28. Linear extensor erythema. Intense erythema involving the anterior aspects of the thigh and tibia, with Gottron's sign over the knees, in a patient with juvenile dermatomyositis.
Figure 29. Linear extensor erythema involving the forearm of a dermatomyositis patient with type III-IV skin, showing partial resolution as hyperpigmentation. Note the erythema at the leading edge, indicative of ongoing activity.
 |
| Figure 30 |
|---|
Figure 30. Linear extensor erythema of the arm in an African American patient, demonstrated as hyperpigmentation. Note the linear hyperpigmentation consistent with pruritus with excoriation, and indicative of ongoing disease activity.
II. A. iii. V-sign rash
Discrete and confluent macular erythema over the lower anterior neck and upper anterior chest. The V-sign rash is associated with dermatomyositis in patients who have Mi-2 autoantibodies, a myositis-specific autoantibody [4].
 |  |
| Figure 31 | Figure 32 |
|---|
Figure 31. V-sign rash. Irregular patchy erythema with prominent telangiectasias in a woman with dermatomyositis.
Figure 32. V-sign rash. Confluent macular erythema involving the face, the neck and extending to form a V-sign rash on the upper chest. Note the sparing in the submental area, a sun protected area. Photosensitivity is thought to play a role in dermatomyositis (see Review).
 |  |
| Figure 33 | Figure 34 |
|---|
Figure 33. V sign rash. Confluent erythema associated with telangiectasia and porcelain white macular areas.
Figure 34. V sign rash. Secondary erosion, hemorrhagic crust and scale on a background of confluent macular erythema involving the neck. Such changes are due to superficial blistering in acute, severe dermatomyositis.
 |
| Figure 35 |
|---|
Figure 35. V sign rash. Erythema and scale in the V of the neck in a boy with juvenile dermatomyositis.
II. A. iv. Shawl sign
Discrete and confluent macular erythema in the distribution of a shawl (i.e., over the upper back, posterior neck, and shoulders, and sometimes extending to the lateral arms). The shawl-sign rash is also associated with dermatomyositis in patients with anti-Mi-2 autoantibodies, a myositis-specific autoantibody. However, this association is less frequent than with the V-sign rash [4].
 |  |
| Figure 36 | Figure 37 |
|---|
Figure 36. Shawl sign. Confluent macular erythema involving the upper back and extending onto the shoulders in a man with dermatomyositis. Higher magnification demonstrates the porcelain white macules that can be seen on a background of erythema.
Figure 37. Shawl sign. Confluent macular erythema involving the upper back and extending onto the shoulders and upper arms in a man with dermatomyositis. Erythema extends to the nape of the neck and into the scalp. Note the demarcation from the rest of the back. This patient's cutaneous findings of dermatomyositis erupted simultaneously with the onset of symptoms of gallbladder carcinoma.
 |
| Figure 38 |
|---|
Figure 38. Shawl sign. Sharply demarcated faint erythema involving the upper back and neck in a Korean man with dermatomyositis and metastatic gastric carcinoma. Note the linear streaks indicative of pruritus and excoriation.
II. A. v. Other sun exposed erythemas
Other erythematous rashes (not mentioned above) in a sun exposed distribution. These findings are indicative of photosensitivity and often present in patients with adult and juvenile dermatomyositis.
 |  |
| Figure 39 | Figure 40 |
|---|
Figure 39. Sun exposed erythema. Macular erythema resolving as hyperpigmentation in a bathing suit distribution, with some extension onto areas of non-sun exposed skin, seen in a woman with underlying malignancy and dermatomyositis.
Figure 40. Sun exposed erythema. Erythema involving the cartilaginous portion of the helix of the ear with sparing of the ear lobe in a girl with juvenile dermatomyositis.
 |  |
| Figure 41 | Figure 42 |
|---|
Figure 41. Sun exposed erythema. Hyperpigmentation and scale with underlying erythema involving the sun-exposed posterior auricular skin in a woman with dermatomyositis.
Figure 42. Sun exposed erythema. Sun exposed erythema with sparing of the upper chest, demonstrating secondary scale, crust and erosions in a woman with dermatomyositis.
II. B. Erythematous lesions in non-sun exposed areas
Erythema in areas not exposed to the sun; i.e., usually covered by clothing or protected areas, including such sites as sub-mental, flexural, palms and soles, trunk, hip and groin. Non-sun exposed erythema is found in addition to or exclusive of sun exposed erythema. It can be seen in patients with adult or juvenile dermatomyositis or polymyositis.
 |  |
| Figure 43 | Figure 44 |
|---|
Figure 43. Non-sun exposed erythema. Confluent macular erythema with linear erosions and crusts secondary to excoriation involving the non-sun exposed skin of the buttocks in a girl with juvenile dermatomyositis. Dermatomyositis may be associated with intense pruritus [3].
Figure 44. Non-sun exposed erythema resulting in hyperpigmentation of the buttocks in an African American patient with type IV skin, representing chronic active or resolving dermatomyositis.
 |  |
| Figure 45 | Figure 46 |
|---|
Figure 45. Sun exposed and non-sun exposed erythema involving the shawl area and lower back, with a wide band of sparing in a man with dermatomyositis. There are also well-demarcated areas of uninvolved skin of the mid back. This patient's cutaneous findings of dermatomyositis erupted simultaneously with the onset of symptoms of gallbladder carcinoma.
Figure 46. Holster sign. Macular erythematous to violaceous rash confined to the lateral thigh or hip with the contour of a gun holster, thus the name "holster sign," in this child with juvenile dermatomyositis.
II. C. Erythroderma
Extensive areas of confluent erythema, involving both sun exposed and non-sun exposed skin in patients with dermatomyositis, defined here as involving greater than 50 percent of the body surface area. In its most severe manifestation, erythroderma may involve the entire body surface area.
 |
| Figure 47 |
|---|
Figure 47. Erythroderma. Composite view showing confluent erythema with scale involving both sun-exposed and non-sun exposed skin in a boy with severe juvenile dermatomyositis. Note the sharp demarcation at the bathing trunk line involving the upper anterior thighs. This child also has severe subcutaneous edema.
III. Vasculopathic Lesions of Dermatomyositis
III. A. Livedo reticularis
A fixed peripheral vascular pattern characterized by a violaceous to erythematous netlike mottling of the skin. This pattern is usually found on the trunk or the proximal extremities. This vascular pattern persists after the skin has been warmed.
 |  |
| Figure 48 | Figure 49 |
|---|
Figure 48. Livedo reticularis. Close up image of livedo reticularis involving the skin of the upper thigh showing a net like pattern of erythema. Livido reticularis can be a cutaneous manifestation of several serious systemic conditions, including dermatomyositis. In some cases focal ulceration secondary to infarction can be seen on a background of livedo reticularis. (see Figure 51 under Ulceration.)
Figure 49. Livedo reticularis manifesting as non-blanchable, netlike erythema of the chest and proximal upper arm in a patient with juvenile dermatomyositis.
III. B. Ulceration
Complete loss of the epidermis, and at least partial loss of the dermis, and possibly the subcutaneous tissue secondary to vascular insufficiency, trauma, pressure, infection, or frank infarction. Depending on the depth of ulceration, healing can result in depressed scarring.
 |  |
| Figure 50 | Figure 51 |
|---|
Figure 50. Ulceration. Four year old girl with juvenile dermatomyositis with deep ulceration involving the skin and subcutaneous tissue of the posterior thigh. The ulcer extends to the biceps femoris and semitendinosus muscles. Note the presence of livedo reticularis on the posterior aspect of both thighs.
Figure 51. Ulceration involving the skin of the elbow with secondary crust and possible infection. The elbow is an anatomic site subject to pressure and trauma. Note the background of poikiloderma in this patient with severe juvenile dermatomyositis.
III. C. Gingival erythema and capillary changes
Erythema of the gingival rim at the base of the dental crown is a result of capillary dilatation. This can result in bleeding and swelling of the gums, focal erosions or aphthae, i.e., shallow ulcerations.
 |  |
| Figure 52 | Figure 53 |
|---|
Figure 52 and Figure 53. Gingival capillary changes. Telangiectasia involving the gingival rim demonstrating a combination of a mat-like pattern, as well as a complex branching pattern of growth.
III. D. Periungual erythema and telangiectasia
Erythema of the skin and dilatation of blood vessels bordering the base and lateral aspects of the nail plate (periungual area). These findings are also referred to as periungual capillary loop changes. Prominent dilated and tortuous blood vessels may be accompanied by vessel drop out, i.e., loss of vascular supply. Involved skin may become atrophic. Periungual erythema and telangiectasia may be seen in either adult or juvenile dermatomyositis or polymyositis, but is also commonly seen in other connective tissue diseases, particularly in systemic sclerosis, mixed connective tissue disease and systemic lupus erythematosus. Similar to progressive systemic sclerosis (scleroderma), in dermatomyositis, the degree of telangiectasia and vessel dropout reflects ongoing disease activity, particularly in the skin [5]. Magnification with the aid of a dermatoscope [6], ophthalmoscope, or otoscope, particularly under a droplet of oil or water, can be helpful in visualizing more subtle changes.
 |  |
| Figure 54 | Figure 55 |
|---|
Figure 54. Periungual telangiectasia. Nailfold capillary loop changes, including dilatation, tortuosity, vessel dropout and mild atrophy with cuticular overgrowth.
Figure 55. Periungual telangiectasia. Telangiectasia with prominent tortuosity and vessel dropout, demonstrated under a droplet of oil in patients with juvenile dermatomyositis. Image shows dramatic vessel tortuosity and disarray, and more discrete hairpin and bushy loops.
 |  |
| Figure 56 | Figure 57 |
|---|
Figure 56 Periungual telangiectasia. Shows hairpin and bushy loops in juvenile dermatomyositis.
Figure 57. Periungual telangiectasia. Under a droplet of oil, more subtle, small, thin vessels demonstrate parallel striations indicative of early telangiectatic change.These changes are associated with cuticular overgrowth in this patient.
III. E. Digital infarction
This relatively rare manifestation of dermatomyositis represents complete vascular occlusion, resulting in tissue necrosis. Causes include thrombosis, vasospasm, vasculitis, or other unknown etiologies, which lead to loss of vascular supply and tissue necrosis.
 |
| Figure 58 |
|---|
Figure 58. Digital infarction. A young woman with refractory dermatomyositis who initially developed painful "blue fingers" in multiple digits. Angiogram (left panel of enlarged image) revealed abrupt occlusion of the medial and lateral palmar digital arteries at the level of the proximal phalanx of the corresponding digits (right panel of enlarged image).
IV. Hand Lesions
IV. A. Mechanic's hands
Hyperkeratotic lesions on the palmar or lateral aspects of the digits, usually at sites of friction, which can include cracking, fissuring, scaling, and hyperpigmentation. These lesions can be easily mistaken for hand dermatitis, but they are often refractory to routine topical therapy. Mechanic's hands can be associated with anti-synthetase autoantibodies, including anti-Jo1, which are myositis-specific autoantibodies [4]. Mechanic's hands are not commonly seen in children with the IIM.
 |  |
| Figure 59 | Figure 60 |
|---|
Figure 59. Mechanic's hands. Scale, fissuring and hyperpigmentation along the lateral aspects of the fingers in this African American patient.
Figure 60. Mechanic's hands. Subtle scale along the lateral aspect of the index finger in association with cuticular overgrowth in this female patient with polymyositis and Jo1 autoantibody.
 |
| Figure 61 |
|---|
Figure 61. Mechanic's hands. Palmar erythema with prominent lichenification (accentuated skin markings), hyperkeratosis and mild scaling in a patient with severe juvenile dermatomyositis. Note the focal blanching over the hypothenar eminence resulting in a mottled pattern. Similar changes can be seen on the plantar surfaces of the feet in patients with severe dermatomyositis.
IV. B. Cuticular overgrowth
Enlargement and overgrowth of the cuticle onto the nail plate. Cuticular overgrowth is commonly seen in adult dermatomyositis patients with Mi-2 autoantibodies and is increased in frequency in adult dermatomyositis patients (30%), regardless of autoantibody status [4].
 |  |
| Figure 62 | Figure 63 |
|---|
Figure 62. Cuticular overgrowth. Overgrowth of the cuticle raised above the nail plate in a boy with juvenile dermatomyositis. Periungual telangiectasia and vessel dropout are also present.
Figure 63. Cuticular overgrowth obscuring the lunula of the nail. Note that the hyperpigmentation also partially obscures the telangiectasia involving the nail bed in this African American patient with dermatomyositis.
IV. C. Palmar and plantar vasculopathy
Palmar and plantar erythematous to violaceous macules and patches may be manifestations of underlying vascular instability in patients with dermatomyositis.
 |  |
| Figure 64 | Figure 65 |
|---|
Figure 64 & 65. Palmar and plantar vasculopathy. Discrete erythematous to violaceous macules can be seen on the palmar surface of the fingers and palm (Figure 64), as well as the plantar aspect of the foot (Figure 65) in the setting of dermatomyositis.
 |  |
| Figure 66 | Figure 67 |
|---|
Figure 66. Palmar vasculopathy. Diffuse macular erythema of the palm and fingers with a mottled pattern (blanching), indicative of vasomotor instability in a patient with juvenile dermatomyositis. Note the deep violaceous hue of erythema involving the lateral aspect of the index finger.
Figure 67. Palmar vasculopathy. Palmar erythema manifesting as fixed erythematous to violaceous slightly depressed plaques with cribriform scarring (close up in insert). Note that these lesions are most prominent over the plantar joint creases.
IV. D. Palmar papules and plaques
Edematous, erythematous to violaceous raised, often tender papules or plaques overlying the joint creases of the palmar hand surface. Biopsy may demonstrate mucin deposition in the dermis.
 |  |
| Figure 68 | Figure 69 |
|---|
Figure 68. Papules and plaques. Erythematous to violaceous papules and plaques on the lateral aspects of the fingers adjacent to or directly overlying joint crease in a middle-aged woman with an overlap syndrome of dermatomyositis and systemic lupus erythematosus.
Figure 69. Palmar papules. Discrete raised flat topped scaly papules on the palmar surface of the fingers in the vicinity of the joint creases.
V. Other Active Lesions
V. A. Subcutaneous edema
Swelling of skin and soft tissue that may be localized or generalized.
 |  |
| Figure 70 | Figure 71 |
|---|
Figure 70. Subcutaneous edema. A patient with juvenile dermatomyositis, who presented with progressive, severe muscle weakness, cardiac dysfunction, and marked generalized subcutaneous edema, shown here involving the arm and trunk. Adult and juvenile dermatomyositis may present with subcutaneous edema, a marker of severe disease [7].
Figure 71. Subcutaneous edema associated with linear extensor erythema involving the forearm in a patient with dermatomyositis.
 |  |
| Figure 72 | Figure 73 |
|---|
Figure 72. Subcutaneous edema. Massive facial edema in man with acute onset dermatomyositis. Note the predilection for the lower eyelids.
Figure 73. Subcutaneous edema. Marked periorbital edema involving both the upper and lower eyelids in a patient with a classic heliotrope rash characteristic of dermatomyositis.
V. B. Panniculitis
Inflammation of the subcutaneous fat, seen uncommonly in adult and juvenile dermatomyositis [8, 9]. Panniculitis, however, is frequently detected subclinically as edema of the subcutaneous tissue on magnetic resonance imaging [10]. Panniculitis may result in subcutaneous dystrophic calcification and/or focal lipoatrophy [10, 11].
 |
| Figure 74 |
|---|
Figure 74. Panniculitis of the left upper arm, which presented as swelling, redness and pain, which eventuated in depressed scars with overlying crust in a Korean woman with dermatomyositis.
V. C. Alopecia
Hair loss. May be diffuse or focal.
V. C. i. Diffuse alopecia
Non-scarring, widespread alopecia. May be multifactorial.
 |  |
| Figure 75 | Figure 76 |
|---|
Figure 75. Diffuse alopecia in young woman with dermatomyositis with a background of erythema.
Figure 76. Diffuse alopecia with erythema and erosions on the scalp in a child with juvenile dermatomyositis.
V. C. ii. Focal alopecia
Focal patchy alopecia with erythema, localized to areas of inflammation. Seen in adult and juvenile dermatomyositis.
 |
| Figure 77 |
|---|
Figure 77. Focal alopecia in juvenile dermatomyositis with associated scale on the scalp.
V. D. Flagellate Erythema
Linear erythematous streaks, usually involving the upper to mid back, which occurs in association with pruritus. Although this occurs in the setting of dermatomyositis [12], it has also previously been reported in association with chemotherapeutic agents.
 |
| Figure 78 |
|---|
Figure 78. Flagellate erythema. Linear erythema in streaks on the back with central sparing in a patient with dermatomyositis. This may be secondary to excoriation.
VI. Chronic Lesions or Lesions of Damage which are Characteristic of Dermatomyositis
VI. A. Atrophy or Dyspigmentation without blanchable erythema in the illustrated lesion
 |  |
| Figure 8 | Figure 9 |
|---|
Figure 8. Atrophy. This cropped picture, showing Gottron's papules, demonstrates evidence of damage at the site of active Gottron's papules, including porcelain white scarring and atrophy over the proximal interphalangeal joint. The surrounding erythema indicates ongoing active disease at this site.
Figure 9. Atrophy. This image of Gottron's papules demonstrates central atrophy and depressed porcelain white scarring with prominent telangiectasia overlying the proximal interphalangeal joints. This is indicative of a combination of chronic activity and damage.
 |
| Figure 79 |
|---|
Figure 79. Atrophy. This cropped image of hypopigmented change in the distribution of Gottron's is indicative of damage. Seen in full under Gottron's sign, Figure 11.
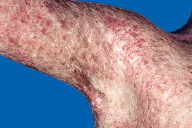
VI. B. Poikiloderma
A mottled pattern of hyperpigmented and hypopigmented macules interspersed with fine telangiectasia and cutaneous atrophy on a background of erythema. This pattern is a manifestation of disease chronicity which can occur in photodistributed or non-photodistributed areas.
 |  |
| Figure 80 | Figure 81 |
|---|
Figure 80. Poikiloderma. Close up of poikiloderma, a mixture of hypo- and hyper pigmentation with telangiectasia atrophy and milia.
Figure 81. Poikiloderma. Extensive poikiloderma, a mixture of hypo- and hyper-pigmentation with telangiectasia and epidermal atrophy at previous sites of active cutaneous dermatomyositis, including the non-sun exposed skin of the buttocks, upper thigh and lower back.
 |
| Figure 82 |
|---|
Figure 82. Poikiloderma. Severe poikilodermatous changes with signs of activity (lichenified papules) and scar with sparing of the axillary vault, suggesting a distribution with photoexacerbation.
VI. C. Calcinosis
Dystrophic calcification involving the skin, subcutaneous tissue, fascia or muscle. This most often occurs as a complication of juvenile dermatomyositis, but may also be seen in patients with adult dermatomyositis, and overlap myositis (particularly overlapping with systemic sclerosis). Cutaneous involvement is not a pre-requisite for the development of calcinosis, which may also occur in polymyositis patients. There are four sub-types: superficial plaques/nodules, tumerous deposits, fascial plane deposition, and exoskeleton; many patients have a combination of two or more subtypes [13].
VI. C. i. Superficial plaques and nodules
Superficial dermal or subcutaneous papules, plaques and nodules that affect areas of potential trauma and inflammation, such as the elbows, knees, and posterior thigh.
 |  |
| Figure 83 | Figure 84 |
|---|
 |  |
| Figure 85 | Figure 86 |
|---|
 |
| Figure 87 |
|---|
Calcinosis, superficial plaques and nodules. Here the lesions are located in the posterior thigh in patients with juvenile dermatomyositis (Figure 83), on the popliteal fossa (Figure 84), the knee (Figure 85), and overlying the buttocks bilaterally (Figure 86). Involvement of anterior neck, as seen in this image (Figure 87) is an unusual finding.
VI. C. ii. Tumorous deposits
Deep dermal and subcutaneous deposition, of calcium in sites of inflammation and subclinical trauma.
 |  |
| Figure 88 | Figure 89 |
|---|
Figure 88. Tumorous calcinosis. Multiple lesions of tumerous calcinosis involve the trunk in a young man with juvenile dermatomyositis. Note several lesions at the axilla that demonstrate superficial ulcerations with extrusion of calcium. Otherwise, the overlying skin appears relatively normal, as seen on the anterior chest and abdomen.
Figure 89. Tumorous calcinosis. Radiographic image demonstrating extensive tumerous dystrophic calcification in the calves of a 10-year-old boy with juvenile dermatomyositis.
VI. C. iii. Fascial plane deposition
Calcinosis deposited along the intermuscular fascial plane. This often presents as a palpable indurated, well-demarcated linear deposit along the fascial planes of the extremities.
 |
| Figure 90 |
|---|
Figure 90. Fascial planar calcification. Dystrophic calcification along the fascial plane of the biceps muscle in a child with juvenile dermatomyositis.
 |  |
| Figure 91 | Figure 92 |
|---|
Figures 91 & 92. Fascial plane calcification. Radiographic images demonstrating extensive fascial planar calcification in the pelvic girdle muscles, pre- and post- tibial and along the dorsal aspect of the foot in an adult woman with a history of juvenile dermatomyositis.
VI. C. iv. Exoskeleton
External calcinosis that extensively encases soft tissues.
 |
| Figure 93 |
|---|
Figure 93. Exoskeleton. Abdominal computed tomography scan demonstrating calcinosis encasing the torso and also involving the psoas muscles in a woman with a history of juvenile dermatomyositis. The calcium appears as a white density.
VI. D. Lipodystrophy
Loss of subcutaneous fat may be localized, partial (in the extremities), or widely distributed (total lipodystrophy). Generalized and sometimes partial lipodystrophy are accompanied by insulin resistance and hyperlipidemia. This occurs most often as a complication of juvenile dermatomyositis in approximately 10 percent of patients and rarely occurs in adult dermatomyositis [11].
VI. D. i. Localized lipoatrophy
Fat loss from localized areas, often resulting in depression or dimpling of the skin surface. Also known as focal lipoatrophy.
 |
| Figure 94 |
|---|
Figure 94. Localized (focal) lipoatrophy. A localized depression due to fat loss involving the upper arm in a patient with dermatomyositis.
VI. D. ii. Partial lipodystrophy
Fat loss of the upper and/or lower extremities, with relative sparing of the trunk.
 |
| Figure 95 |
|---|
Figure 95. Partial lipodystrophy. A young woman with a longstanding history of juvenile dermatomyositis with partial lipodystrophy in the lower extremities. Note the sculptured appearance of the calves following the lines of the musculature, indicative of fat loss.
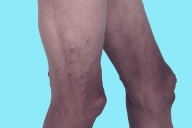
VI. D. iii. Generalized lipodystrophy
Generalized loss of fat in the face, trunk, abdomen, and all extremities.
 |  |
| Figure 96 | Figure 97 |
|---|
Figure 96. Generalized lipodystrophy with widespread loss of fat from the extremities and trunk in an adolescent with juvenile dermatomyositis. Note generalized hyperpigmentation as well as acanthosis nigricans at the neckline and axilla. This patient also has subcutaneous calcifications, which are associated with generalized lipodystrophy in juvenile dermatomyositis [11].
Figure 97. Generalized lipodystrophy. Close up of generalized lipodystrophy involving the lower extremities in an adolescent with juvenile dermatomyositis. Note the vague livedo pattern and the focal extrusions of calcium.
 |  |
| Figure 98 | Figure 99 |
|---|
Figure 98. Generalized lipodystrophy. Loss of fat from the buccal fat pad in a young woman with juvenile dermatomyositis and total lipodystrophy. Buccal fat loss is frequently present in both generalized and partial lipodystrophy associated with juvenile dermatomyositis [11].
Figure 99. Generalized lipodystrophy. T1 axial magnetic resonance image of thighs demonstrates almost complete absence of the subcutaneous fat surrounding the muscle tissue in this patient with juvenile dermatomyositis and generalized lipodystrophy. Normally, subcutaneous fat would appear as a rim of white tissue surrounding the thigh muscles. Note preservation of the fat in the bone marrow.
VI. E. Acanthosis nigricans
Manifested as velvety hyperpigmented papillomatous papules and plaques involving the intertriginous areas, acanthosis nigricans can be associated with the endocrinopathies occurring in patients with inflammatory myopathies. In this setting, these include either isolated insulin resistance or insulin resistance associated with lipodystrophy.
 |  |
| Figure 100 | Figure 101 |
|---|
Figure 100. Acanthosis nigricans. Prominent acanthosis nigricans is seen here in the axilla of a patient with juvenile dermatomyositis and generalized lipodystrophy.
Figure 101. Acanthosis nigricans. Acanthosis nigricans involving the neck in the same patient in Figure 100.
VI. F. Depressed scar
End stage lesions manifested as depressions due to atrophy or fibrosis. May be the result of ulceration, vascular insufficiency seen in Raynaud's, panniculitis, infection, or other persistent inflammatory manifestations.
 |  |
| Figure 50 | Figure 102 |
|---|
Figure 50. Depressed scar. Punched out scarring on the thighs in association with deep ulceration in a patient with juvenile dermatomyositis. Note the background livedo pattern.
Figure 102. Depressed scar. Pock like scarring near the medial canthus in an area where erosions were previously observed as part of an active dermatomyositis lesion. Heliotrope rash and malar erythema are also present.
VII. Overlap Cutaneous Manifestations of Collagen Vascular Diseases in the Setting of Myositis
The following slides represent several examples of dermatomyositis or polymyositis with overlap features of systemic sclerosis.
VII. A. Sclerodactyly
Tapering of the fingertips with loss of the distal pulp, which is most often present in the limited form of systemic sclerosis, but which can also be seen in the IIM when overlapping with systemic sclerosis.
 |
| Figure 103 |
|---|
Figure 103. Sclerodactyly. Loss of distal pulp with tapering of the fingertips, most likely related to digital infarctions in a woman with a history of juvenile dermatomyositis, who has PM-Scl autoantibodies. Note the Gottron's papules overlying the interphalangeal joints.
VII. B. Sclerodermatous changes
 |  |
| Figure 104 | Figure 105 |
|---|
Figure 104. Birdlike-faces (beaked nose and radial furrowing around the mouth), a feature of systemic sclerosis, is present in a woman with a history of juvenile dermatomyositis and limited systemic sclerosis, including calcinosis, Raynaud's and sclerodactyly. This patient has PM-Scl autoantibodies, supporting the diagnosis of an overlap syndrome of systemic sclerosis and dermatomyositis.
Figure 105. En coup de sabre. This patient with juvenile dermatomyositis demonstrates overlap features of en coup de sabre, a feature of localized scleroderma.
ACKNOWLEDGEMENTS: We thank Drs. Edward Cowen and Heidi Kong for critical reading of the manuscript.
Appendix. The following members of the International Myositis Assessment and Clinical Studies (IMACS) Group have also contributed photographs for use in the photoessay: Suzanne Bowyer, MD, University of Indiana, Indianapolis, IN; Brian M. Feldman, MD, University of Toronto, Toronto, Ontario, Canada; Katalin Danko, MD, University of Debrecen, Debrecen, Hungary; Ignacio Garcia de la Torre, MD, University of Guadalajara, Guadalajara, Mexico; Bianca Lang, MD, IWK Health Centre and Dalhousie University, Halifax, Nova Scotia, Canada; Carol Lindsley, MD, University of Kansas, Kansas City, KS; Peter Malleson, MD, University of British Columbia, Vancouver, British Columbia, Canada; Lauren M. Pachman, MD, Children's Memorial Hospital, Chicago, IL; Paul H Plotz, MD, National Institute of Arthritis and Musculoskeletal and Skin Diseases, (NIAMS), NIH, Bethesda; MD; Robert Rennebohm, MD, Ohio State University, Columbus, OH; Jacques Serratrice MD, Hôpital de la Timone, Marseilles, France; David Sherry, MD, Children's Hospital, University of Washington, Seattle, WA; Richard Sontheimer, MD, University of Oklahoma, Oklahoma City, OK; Yeong Wook Song, MD, Seoul National University, Seoul, Korea; Ira Targoff, MD, Oklahoma Medical Research Foundation, Oklahoma City, OK; Maria Turner, MD, National Cancer Institute, National Institutes of Health, Bethesda, MD; Jiri Vencovsky, MD PhD, Institute of Rheumatology, Prague, Czech Republic; Carlos A. von Muhlen, MD, Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil; Victoria P. Werth, MD, University of Pennsylvania, Philadelphia, PA; Robert Wortmann, MD, Dartmouth Medical School, Hanover, NH
Others contributors to the slide collection include:
Joseph Lopreiato, MD, MPh, Uniformed Services University of the Health Sciences, Bethesda, MD; Laura James-Newton, PhD RN, National Institute of Environmental Health Sciences, National Institutes of Health, Bethesda, MD; Sheila Oliveira, MD, Sao Paolo, Brazil; Kazuki Takada MD, NIAMS, NIH, Bethesda, MD; Carol Wallace, Children's Hospital, University of Washington, Seattle, WA
References
1. Huber AM, Dugan EM, Lachenbruch PA, Feldman BM, Perez MD, Zemel LS, Lindsley CB, Rennebohm RM, Wallace CA, Passo MH, Reed AM, Bowyer SL, Ballinger SH, Miller FW, Rider LG. Reliability of the Cutaneous Assessment Tool (CAT) in Juvenile Dermatomyositis. Rheumatology. 2007; 46 (10): 1606- 1611. [PubMed]2. Miller FW, Rider LG, Chung YL, Cooper R, Danko K, Farewell V, Lundberg I, Morrison C, Oakley L, Oakley I, Pilkington C, Vencovsky J, Vincent, K, Scott DL, Isenberg DA, for the International Myositis Outcome Assessment Collaborative Study Group. Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology. 2001; 40: 1262-1273. [PubMed]
3. Hundley JL, Carroll CR, Lang W, Snively B, Yosipovitch G, Feldman SR, Jorizzo JL. Cutaneous symptoms of dermatomyositis significantly impact patients' quality of life. J Am. Acad Dermatolol. 2006; 54 (2): 217-20. [PubMed]
4. Love, LA, Leff, RL, Fraser, DD, Targoff, IN, Dalakas, M, Plotz, PH, Miller, FW. A New Approach to the classification of idiopathic inflammatory myopathy: myositis-specific auto-antibodies define useful homogeneous patient groups. Medicine, Baltimore, 1991; 70(6): 360-74. [PubMed]
5. Christen-Zaech S, Seshadri R, Sundberg J, Paller A, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in juvenile dermatomyositis. Arthritis Rheum. 2008; 58 (2): 571-76. [PubMed]
6. Sontheimer RD, A portable digital microphotography unit for rapid documentation of periungual nailfold capillary changes in autoimmune connective tissue diseases. J Rheumatol. 2004 Mar;31(3):539-44. [PubMed]
7. Poitras JM, Dennis GJ, and Rider LG. Juvenile dermatomyositis presenting with anasarca. J Pediatr. 2000; 138: 942-45. [PubMed]
8. Solans R, Cortes J, Selva A, Garcia-Patos V, Jimenez FJ, Pascual C, Bosch J, Vilardell M. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatoll. 2002; 46 (5 Suppl): S148-S150. [PubMed]
9. Ghali FE, Reed AM, Groben PA, McCauliffe DP. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999; 16 (4): 270-72. [PubMed]
10. Kimball AB, Summers RM, Turner M, Dugan E, Hicks J, Miller FW, and Rider LG. Magnetic resonance imaging detects occult skin and subcutaneous abnormalities in juvenile dermatomyositis: Implications for diagnosis and therapy. Arthritis Rheumatism. 2000; 43:1866-73. [PubMed]
11. Bingham A, Mamyrova G, Rother KI, Oral E, Cochran E, Premkumar A, Kleiner D, James-Newton L, Targoff IN, Pandey JP, Mercatante Carrick D, Sebring N, O'Hanlon TP, Ruiz Hidalgo M, Turner M, Gordon LB, Laborda J, Bauer SR, Blackshear PJ, Imundo L, Miller FW, Rider LG, for the Childhood Myositis Heterogeneity Study Group. Predictors of Acquired Lipodystrophy in Dermatomyositis and a Gradient of Severity. Medicine.2008; 87: 70- 86. [PubMed]
12. Noussari HC, Ha VT, Laman SD, Provost TT, Tausk FA. "Centripetal flagellate erythema": A cutaneous manifestation associated with dermatomyositis. J Rheumatol. 1999; 26 (3): 692- 695. [PubMed]
13. Blane CE, White SJ, Braunstein EM, Bowyer SL, Sullivan DB. Patterns of calcification in childhood dermatomyositis. AJR. 1984; 142: 397 - 400. [PubMed]
© 2009 Dermatology Online Journal

