Abstract
Objectives
HIV-1-infected persons spontaneously controlling viremia without treatment (SCV) are rare. Gender and race effects on prevalence and outcome are poorly defined, and it is unclear whether SCV qualitatively or quantitatively differs from typical infection. These issues are examined.
Design
Medical records of 46,524 persons receiving outpatient care for HIV-1 infection were reviewed. Of these, 29,811 had adequate viremia testing for SCV screening.
Methods
SCV was defined as ≥3 consecutive plasma viremia measurements <50 RNA copies/mL spanning ≥1 year without treatment. SCV loss was defined as ≥3 consecutive viremia measurements ≥50 or one ≥1,000. Demographics of persons with SCV were compared to the total population. Viremia and blood CD4+ T cell levels during SCV were compared between demographic subgroups and persons who maintained or lost SCV during observation.
Results
53 persons (0.18%) met SCV criteria. Prevalence was higher for women versus men and Blacks versus Whites; these appeared independent. Loss of SCV was observed at 1.22%/year, and significantly associated with viremia “blips.” Blip magnitudes fit log-normal distributions with means below 50 RNA copies/mL.
Conclusions
Our novel observation of higher SCV prevalence in women and Blacks is consistent with prior studies of typical chronic infection. Viremia blips correspond to greater risk of loss of SCV, likely reflecting higher set-point viremia under the limit of detection. Our findings suggest that SCV represents an extreme along a continuum of HIV-1 infection, and not qualitative difference.
Keywords: HIV-1, Elite Controller, Viremia, CD4+ T-cell
INTRODUCTION
Persons with untreated HIV-1 infection typically progress to AIDS at rates proportional to “set-point” plasma viremia [1, 2], but there are rare individuals (often termed “elite controllers” [3, 4]) with spontaneous control of viremia (SCV) without antiretroviral therapy. While viral genetic defects have been reported [5–8], the majority are infected with competent viruses [7, 9, 10] and SCV is attributable to poorly understood immune factors [3]. Genetic screenings have consistently identified HLA-I as the major determinant of SCV [11, 12], although a third or more of persons with SCV have no alleles known to be associated with protection [13].
The prevalence, demographics, and long-term outcome of SCV are poorly defined. Beyond HLA-I, the role of race is unknown. Many SCV cohorts have been predominately men, and the role of gender is undefined. Finally, few data address the durability of SCV, and influence of the abovementioned factors is underexplored. Here we assess the frequency, demographics, and outcomes of 53 persons with SCV identified within a group of 29,811 demographically diverse HIV-1-infected persons.
MATERIALS AND METHODS
Subjects, definition of SCV, and loss of SCV
46,524 AIDS Healthcare Foundation (AHF) electronic medical records (Centricity, General Electric Healthcare) from the Los Angeles and Miami metropolitan areas (23,679 and 22,845 respectively) were scanned for persons with three or more plasma viremia measurements spanning at least a year, resulting in 29,811 persons. These were screened for HIV-1-infected persons with SCV defined as three or more consecutive plasma viremia measurements <50 RNA copies/mL spanning at least a year in the absence of ART [14, 15]. This screening included all patients in care at AHF clinics (solely outpatient care), mostly to public patients without private insurance. Records were reviewed for HIV-1 serologies, viremia, CD4+ T cell measurements, viral hepatitis B and C serologies, and prescribed medications, all information from the routine care of patients in the clinics. Date of study entry was the initial date of care at AHF. Estimated duration of HIV-1 infection was conservatively calculated as time from first reported positive HIV-1 serology. Loss of SCV was defined by three consecutive plasma viremia measurements ≥50 RNA copies/mL or a single measurement ≥1000 copies/mL. Isolated viremia measurements ≥50 RNA copies/mL not meeting the definition of SCV loss (non-consecutive) were considered “blips.” Follow-up was censored at time of loss of SCV, initiation of ART (not considered loss of SCV), or last available viremia measurement.
Statistical comparisons
Comparisons between 1) persons with versus without SCV and 2) persons with SCV who remained aviremic versus those who became viremic during follow up were performed using Wilcoxon test and Chi-square or Fisher’s exact tests. For the SCV group, Kaplan-Meier survival curves were generated for time to loss of SCV, including comparisons between subgroups of race, age, and gender using log-rank tests. Cox proportional hazards modeling for time to viremia was performed including age at infection, Hepatitis C Virus (HCV) infection status, race, and gender as covariates. Rate of blood CD4+ T cell level decline during the observation period was estimated using linear mixed effects modeling with random intercept and fixed effect covariates of age at time of infection, initial blood CD4+ T cell level, duration of infection at entry, race, gender, viremic status (persistent SCV versus breakthrough viremia), follow-up time, and the interaction of each covariate with follow-up time, with age at time of infection, initial blood CD4+ T cell level, and duration of infection at entry, centered at the mean. All analyses were conducted using SAS 9.4 (Cary, NC, U.S.A.) or Stata 12 (College Station, TX, U.S.A.).
Normal distribution model for viremia measurements
Viremia measurements <50 RNA copies/mL plasma (<1.7 log10 units) were considered censored. Measurements were assumed to be reasonably independently normally distributed, based on several studies that “elite controllers” generally have very similar mean viremia levels in the range of 1 to 5 RNA copies/mL plasma, which is a much smaller range than the variability of viremia measurements within persons with typical chronic infection [16–19]. The procedure tobit (Stata 12) was used to make maximum likelihood estimates for the mean and standard deviation of combined viremia measurements for persons with sustained versus non-sustained SCV during observation. For each of these datasets the full density and the histogram for uncensored observations were graphed together. The histogram was scaled to set its area equal to the area of the corresponding normal density above 1.7 log10 units viremia.
Institutional review board approval
The study was reviewed and approved by the Biomedical Research Institute of America (BioMed IRB), San Diego, California.
RESULTS
Demographics of persons with HIV-1 infection and spontaneous control of viremia (SCV)
Of 46,524 medical records of HIV-1-infected persons reviewed, 29,811 had adequate viremia measurements for screening, of which 53 (0.18%) were identified as persons with SCV (three consecutive plasma HIV-1 RNA tests <50 copies/ml spanning at least a year without ART, Supplemental Figure S1 and Supplemental Table S1). 26 (49%) were female, 24 (45%) were male, and 3 (6%) were transgender (male to female). The group included 33 (62%) Black, 17 (32%) White (including 4 Latino), 1 (2%) Asian, and 2 (4%) other/unspecified races. Of 26 self-reported routes of transmission, 24 (45%) were sexual, and 2 (4%) were via intravenous drug use. Of 18 screened for HLA B*5701, 2 (11%) had the allele, including 2 of 7 (29%) Whites, 0 of 10 Blacks, and 0 of 1 Asian. Of 47 subjects tested for HCV-reactive antibodies, 8 were seropositive, of which 5 had detectable plasma HCV RNA, and an additional person had detected plasma HCV RNA without serologic testing.
At beginning of observation, median age was 44.8 years (interquartile range, IQR, 36.0 – 50.4) and minimum duration of infection (using date of infection conservatively estimated as first reported positive HIV-1 serology) was a median of 2.2 years (IQR 0.03 – 11.8). Median age at the estimated time of HIV-1 infection was 35.1 years (IQR 27.1 – 42.4). Mean blood CD4+ T cell level at study entry was 882 cells/µL (S.D. 344, range 279–1863). Follow-up during SCV was a median of 3.6 years (IQR 2.3 – 7.6). During observation, 9 persons developed viremia after a mean of 6.1 years (S.D. 3.5, range 1.5 – 11.3) and 5 persons initiated antiretroviral therapy without preceding development of viremia.
Frequency of SCV differs by gender and race
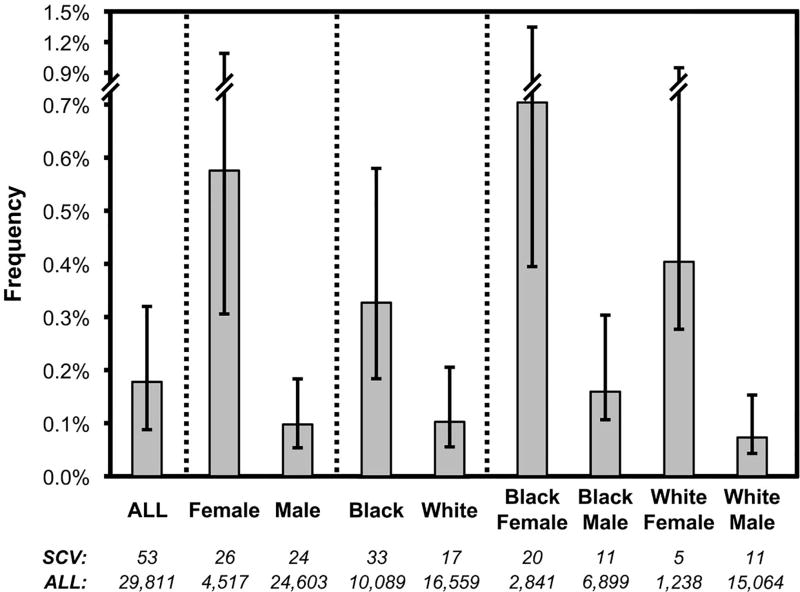
SCV prevalence, calculated from the 29,811 persons screened, varied by gender and race (Figure 1). Women were significantly more likely to have SCV than men (26/4,517=0.58% versus 24/24,603=0.10%, chi-square p<0.001). Blacks were more likely to have SCV than Whites (33/10,089=0.33% versus 17/16,559=0.10%, p<0.001). These two factors appeared independent; highest frequency was among Black women (20/2,841=0.70%), and lowest frequency was among White men (11/15,064=0.07%), with White women (5/1,238=0.40%) and Black men (11/6,899=0.16%) intermediate. Pairwise comparisons between these four groups were statistically significant for Black women versus White men (p<0.0001), Black women versus Black men (p<0.0001), and White women versus White men (p=0.0004). These data suggest that gender and race are determinants of SCV prevalence.
Figure 1. Frequencies of SCV across different demographics of HIV-1 infected persons.
The frequencies of persons meeting SCV criteria in relationship to the total numbers of persons screened for each of the indicated groups are plotted. Numbers are indicated below each bar. Only Black and White race and female and male gender are shown, given the small numbers of other races and transgender persons. The bars indicate 90% confidence intervals (Jeffreys intervals).
Progression to viremia typically occurs over years and may vary by demographic group
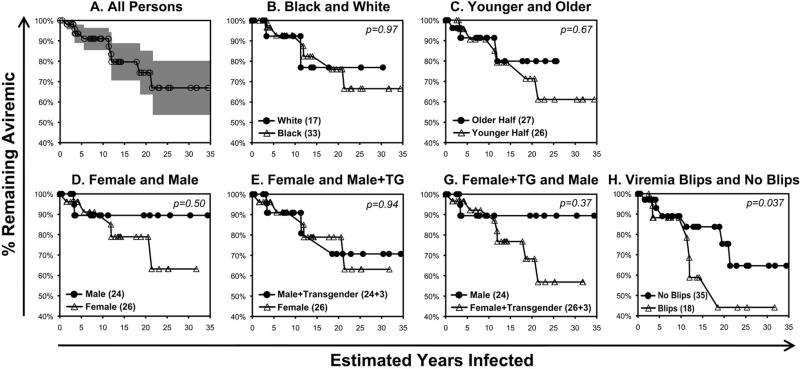
Nine persons lost SCV during observation off ART; median time to loss was 5.5 years (IQR 3.4 – 9.4). The rate of loss was relatively linear over the estimated duration of infection (Figure 2A), corresponding to 1.22% per year, or a half-life of 40.8 years. Black and White races appeared similar with rates of 1.20%/year versus 1.03%/year (Figure 2B). Comparing halves of younger versus older estimated ages at time of infection (Figure 2C, mean age 26.9 ± 4.4 versus 44.3 ± 7.1 years respectively), there was a statistically insignificant trend for faster loss of SCV in the younger versus older subgroup. Comparing genders (Figure 2D, excluding the three male to female transgender persons), there was a larger but statistically insignificant difference for faster progression in women versus men (1.38%/year versus 0.53%/year, p=0.50 by log-rank test). This difference was reduced if the three transgender persons (two of three of whom had observed loss of SCV) were included in the male group (Figure 2E), and increased if they were included in the female group (Figure 2F) although differences remained statistically insignificant. In the Cox regression model, adjusting for estimated age at infection, HCV status, Black versus White race, and gender, transgender (male to female) status was significantly associated with faster progression compared with native female gender (hazard ratio 24.3, p=0.037). Finally, considering the 18 persons with observed “blips” of plasma viremia ≥50 RNA copies/mL versus 35 without (Figure 2G), those with blips had significantly greater progression (−2.27%/year versus −1.23%/year, p=0.04 by log-rank test).
Figure 2. Progression to viremia among 53 persons with SCV.
Kaplan-Meier curves for maintenance of SCV in relationship to estimated years of infection are plotted, defining loss of SCV defined as a single plasma viremia measurement >1000 RNA copies/mL or three consecutive plasma viremia measurements >50 RNA copies/mL. Persons lost to follow-up or started on antiretroviral therapy during SCV were censored. Y-intercepts for all linear regressions were set at 100%.
A. The plot for the entire group is given, with shading of the 80% confidence interval. Linear regression showed r2=0.93, slope = −1.22%/year.
B. The plots for persons identified as Black (33 persons) versus White (17 persons) are given. Linear regressions showed r2=0.93, slope = −1.20%/year versus r2=0.71, slope = −1.03%/year respectively.
C. The plots for the younger (26 persons, mean age 26.9 ± 4.4, range 17.4 to 34.7 years) versus older (27 persons, mean age 44.3 ± 7, range 35.1 to 62.5 years) halves of the group are given. Linear regressions showed r2=0.93, slope = −1.37%/year versus r2=0.79, slope = −0.98%/year respectively.
D. The plots for the women (26 persons) versus men (24 persons) excluding transgender persons are given. Linear regressions showed r2=0.92, slope = −1.38%/year versus r2=0.29, slope = −0.53%/year respectively.
E. The plots for the women including transgender men to women (26 and 3 = 29 persons) versus men (24 persons) excluding transgender persons are given. Linear regressions showed r2=0.94, slope = −1.58%/year versus r2=0.29, slope = −0.53%/year respectively.
F. The plots for women (26 persons) versus men including transgender men to women (24 and 3 = 27 persons) are given. Linear regressions showed r2=0.92, slope = −1.38%/year versus r2=0.85, slope = −1.10%/year respectively.
G. The plots for persons with detectable (≥50 RNA copies/mL plasma) “blips” of viremia (18 persons) versus those without (35 persons) are given. Linear regressions showed r2=0.85, slope = −2.27%/year versus r2=0.93, slope = −1.23%/year respectively (p=0.040).
Higher frequency of intermittently detectable viremia “blips” is associated with loss of SCV
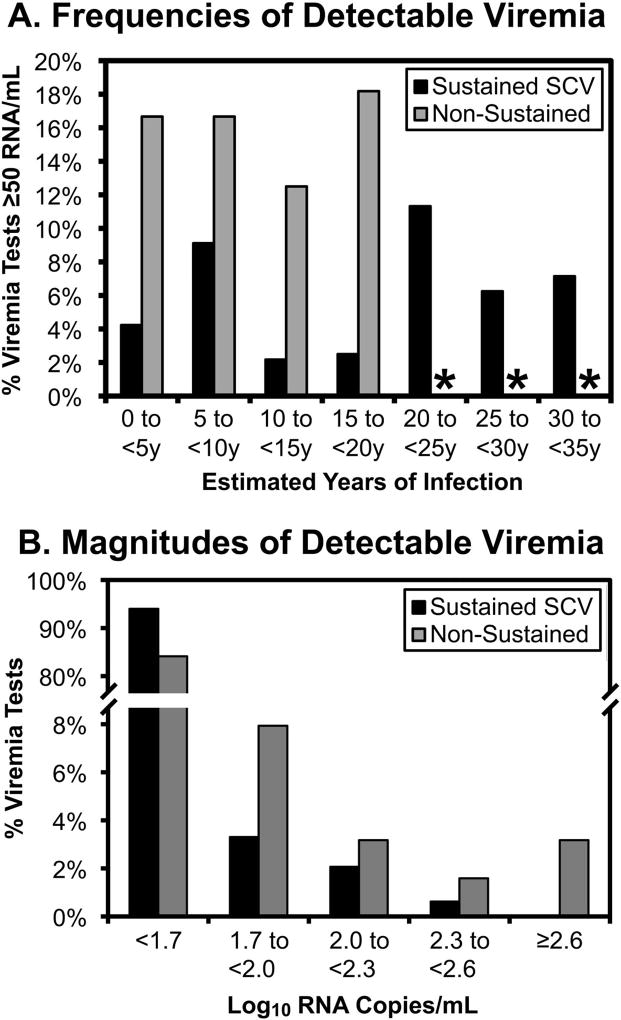
Because the definition of SCV is arbitrarily based on a limit of detection, “blips” of plasma viremia ≥50 RNA copies/mL were assessed. Comparing those with sustained SCV (44 persons) versus those who lost SCV (9 persons) during observation, blip magnitudes were not obviously different (Supplemental Table S2 and Figure 3A versus 3B) or rising before loss of SCV (Figure 3C). However, blip frequencies were generally lower for the sustained SCV group, observed in 5.8% (28/484) versus 15.9% (10/63) viremia measurements (Supplemental Table S2). This difference appeared consistent across five-year intervals after estimated time of HIV-1 infection (Supplemental Table S2 and Figure 4A), and blip frequency did not appear to accelerate before loss of SCV (Supplemental Table S3).
Figure 3. Magnitudes of intermittently detectable viremia during SCV.
Values for viremia testing demonstrating detected “blips” of viremia during SCV are plotted over estimated duration of infection for those with sustained SCV (A) versus non-sustained SCV (B) during the observation period. For those with non-sustained SCV, values are also plotted in relationship to the time of loss of SCV (C), defined as a single value >1000 or three values >50 RNA copies/mL plasma. Viremia measurements before qualification of SCV were excluded, to avoid tests before stable viremia was established in chronic infection.
Figure 4. Frequencies of “blips” of detectable viremia during SCV.
A. The frequencies of detectable viremia tests (≥50 RNA copies/mL plasma) are plotted for persons with sustained (black bars) versus non-sustained (gray bars) SCV for five-year intervals after estimated time of infection. B. The frequencies of blips according to magnitudes are plotted for both groups. Viremia measurements before qualification of SCV were excluded, to avoid tests before stable viremia was established in chronic infection.
*Not plotted because <6 viremia tests were available.
Blip magnitudes suggested set-point viremia levels below 50 RNA copies/mL plasma
Blip magnitude distributions of both groups were consistent with the tails of log-normal curves censored at the limit of detection (Figure 4B), in agreement with the known log-normal distribution of plasma viremia in general [1, 2]. By censored curve fitting to a normal distribution (Supplemental Figure S2), the estimated means of viremia for the sustained and non-sustained SCV groups were 4.5 and 6.7 RNA copies/mL plasma respectively (not statistically significantly different), consistent with biological observations of persistent low viremia in persons with SCV [16–19]. These data suggested that blips reflected variation around stable mean values below 50 copies/mL.
Blood CD4+ T cell level showed overall stability in persons during SCV (without ART)
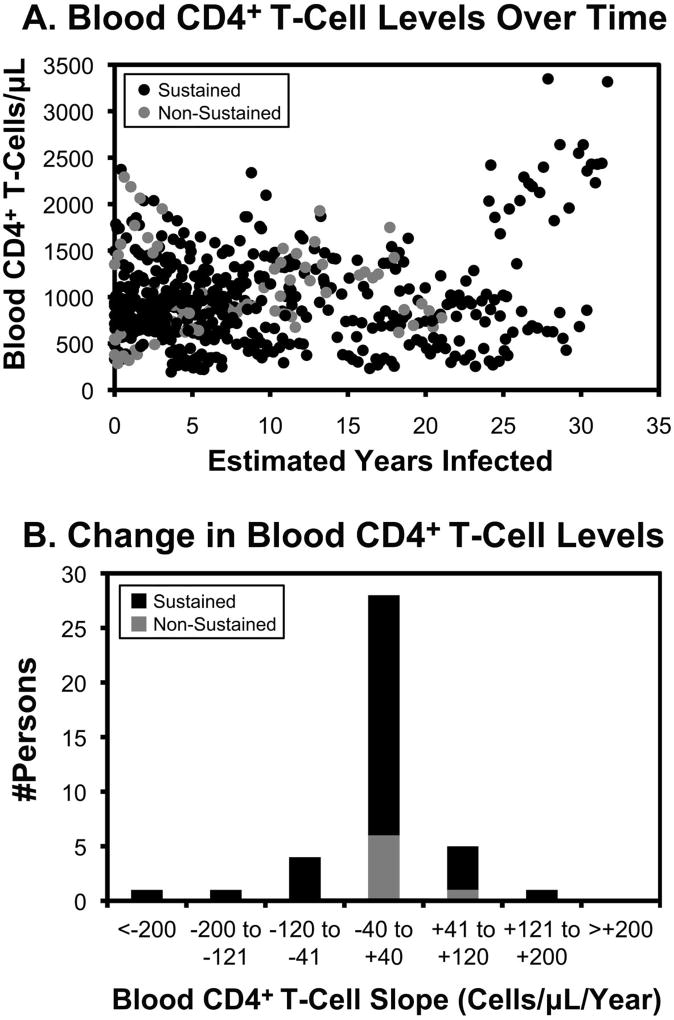
Counts across all persons plotted over estimated duration of infection showed no clear overall pattern over time (Figure 5A). For the 40 subjects with serial observations spanning at least two years of SCV without ART (33 and 7 persons with sustained versus non-sustained SCV during observation respectively), slopes of blood CD4+ T cell levels varied with a normal distribution centered near 0 cells/µL/year, without a clear difference between those observed to sustain versus lose SCV during observation (Figure 5B). Comparisons of women versus men and Blacks versus Whites also showed no significant differences (not shown).
Figure 5. Blood CD4+ T cell levels in persons with SCV over time.
A. Peripheral blood CD4+ T-cell levels are plotted over time for all persons during SCV follow-up (before loss of SCV in the case of those with non-sustained SCV) for the 40 persons with at least two years of measurements during SCV (33 with sustained SCV during observation and 7 with loss of SCV during observation). B. The distributions of observed slopes of blood CD4+ T-cell levels are indicated. The mean values for those with sustained versus non-sustained SCV were −3.0 and −18.7 cells/µL/year respectively.
In a mixed effects model (excluding the three transgender subjects) including all CD4+ T cell observations during SCV, the overall slope for change in CD4+ T cell count (assuming mean values in the cohort for age at estimated time of infection, baseline blood CD4+ T cell level, duration of infection, White race, and male gender) was slightly negative at −9.8 cells/µL/year, (Supplemental Table S4). The slope was more negative by −17.7 in the non-sustained SCV group compared to the SCV group, although this difference was not statistically significant. Higher initial blood CD4+ T cell levels at entry and older age at infection were significantly associated with more negative slopes (by −0.02, p=0.008, and −1.9, p<0.0001, respectively). There was no significant change in slope by race or gender.
DISCUSSION
Studies of persons with SCV (“elite control”) are challenging given the widely varying definitions of SCV, rarity of SCV, and heterogeneity of these persons in terms of factors such as gender, race, co-infections, route of infection that could influence prevalence and outcome of SCV, and monitoring. Prior reports have examined isolated persons and not the prevalence of SCV in demographic context, with few exceptions (Supplemental Table S5). Olson et al [15] compared various definitions of “elite control” in over 17,000 persons (from Europe, Canada, Australia, and sub-Saharan Africa) followed from time of seroconversion. Their three most stringent definitions included one similar to ours (three consecutive plasma viremia measurements <75 RNA copies/mL spanning >one year, without “blips” >1000). Grabar et al [20] examined SCV in >27,000 French patients under care for chronic infection, using a more limiting definition of asymptomatic ART-naïve persons infected at least 10 years with 90% of viremia measurements <500 RNA copies/mL and the most recent measurement <50 RNA copies/mL. Lambotte et al studied SCV from a partially overlapping French cohort of 2,851 persons using a similar definition. Okulicz et al [14] described SCV in a cohort of over 4,400 U.S. persons under care for chronic infection, defining SCV as ≥3 undetectable plasma viremia measurements using varying sensitivities of 400, 200, or 50 RNA copies/mL, spanning at least a year.
Across these four studies, the overall prevalence of SCV varied (Supplemental Table S5). Using definitions of SCV similar to ours, Olson et al and Okulicz et al reported prevalences of 0.55% and 0.56%, and using more stringent criteria Grabar et al and Lambotte et al reported 0.25% and 0.53%, compared to our lower rate of 0.18%. Explanations for this discrepancy might include our lower limit of viremia detection (50 RNA copies/mL) compared to theirs (ranging from 75–500 copies/mL), differences in patient enrollment (i.e. longer duration of infection before enrolling for care at AHF versus the other cohorts) or monitoring (frequency of viremia measurements), or demographic differences between cohorts. A cross-sectional study of single measurement viremia set-point levels in 330 persons about 10 months after infection quoted rates of 0.3% (1/330 persons) below 20 RNA copies/mL or 3.6% (12/330 persons) below 200 RNA copies/mL [2], suggesting that our lower plasma viremia cutoff contributed in part to our lower prevalence of SCV.
Known differences in the pathogenesis of infection based on gender and race would suggest that SCV varies according to these characteristics. To our knowledge, our data provide the most detailed breakdown of SCV prevalence by gender and race available. We observe significantly higher prevalence of SCV among women (0.38%) versus men (0.062%). Olson et al [15] and Okulicz et al [21] did not specify SCV prevalence in women versus men, but contain enough gender information to infer female versus male rates as 0.92% versus 0.44% and 1.18% versus 0.49% respectively (Supplemental Table S5), in agreement with our findings and also consistent with multiple studies showing that viremia is generally lower in women than men [22–27]. We also find SCV prevalence may be higher in Blacks than Whites, 0.19% versus 0.07% respectively. Okulicz et al [21] did not observe this difference (0.55% versus 0.66% respectively), although their study differed in SCV criteria and smaller sample size (Supplemental Table S5). Our finding is consistent with reported lower viremia levels in Blacks versus Whites with chronic HIV-1 infection [26–28]. Considering gender and Black/White race, these appear to be independent factors in prevalence of SCV (Figure 1). Again, a potential caveat is the duration of infection before presenting for care; for example we cannot exclude that earlier presentation of women or Blacks contributes more observation of SCV compared to men or Whites respectively.
Few studies have defined durability of SCV. Olson et al [15] depicted a figure showing 52 of 95 persons losing SCV between ~2 to ~ 22 years of follow-up but did not explicitly provide survival data for loss of SCV; our examination of their data (censored survival) indicates a linear drop-off with a slope of −4.25% per year (r2=0.90, not shown). This is greater than our rate of −1.22% per year, perhaps because they followed persons from seroconversion and therefore included shorter duration SCV that may have been missed in our cohort; alternatively they could have overestimated SCV loss if asymptomatic persons were more likely to drop out of follow-up (to be censored).
Several studies have noted intermittent “blips” of detectable viremia in “elite controllers,” which we also observed. Because our definition for SCV loss includes consecutive detectable viremia measurements, it is not surprising that significantly fewer blips were observed in persons with sustained versus non-sustained SCV, and persons with no observed blips versus observed blips during observation exhibited significantly slower progression to loss of SCV. More interestingly, while we did not observe statistically significantly different blip magnitudes in these comparisons, the magnitudes appeared to fit censored log-normal distributions with means below the limit of detection. Our estimates are consistent with prior reports that “elite controllers” have persistent plasma viremia averaging 1 to 5 RNA copies/mL [16–19], and suggest that blips do not reflect intermittent viral replication in the setting of quiescent infection, but rather represent persistent viremia with normal biologic and/or assay variability for spanning the limit of detection. This strongly suggests that persons with SCV are not qualitatively different, but rather represent an extreme in the continuum of log-normally distributed plasma viremia set-point levels (which are inversely correlated to rate of disease progression) across persons with untreated HIV-1 infection [1, 2].
To our knowledge, our report is the first to examine loss of SCV comparing genders and races. Unfortunately, small numbers limited statistical power for such comparisons. However, the observation that women lost SCV more rapidly then men is consistent with reports that women progress more rapidly than men despite lower set-point viremia levels in early infection [22–24, 26, 27]. We did not note a difference between Blacks and Whites, however, despite reports suggesting more rapid progression in the former [26–28]. Unfortunately, there have been too few Asians in studies of SCV to estimate prevalence or outcomes in that race, and there are only rare descriptions of SCV in East and South Asians [29, 30] despite the large numbers of persons infected in China and India. Okulicz et al [14] reported a rate of 0/73, and we found 1/601 (0.017%), perhaps suggesting that SCV is rarer in Asians. Associations of HLA-I types with disease outcome have been heavily weighted towards persons of White (European) and Black (African) ancestries, limiting statistical power to identify HLA associations for prevalent HLA-I types in other races such as Asians [31]. Indeed while other studies of persons with SCV have observed the “protective” HLA B*5701 type at frequencies from 44 to 85% [8, 13, 32], only 2 of 18 persons tested in our cohort had this type, both White.
Finally, data on the long term clinical outcomes for SCV are somewhat limited, but generally agree that disease progression is relatively slow or absent consistent with the known inverse relationship between “set-point” viremia level and rate of progression to AIDS [1, 2]. The reported rate of blood CD4+ T cell loss in persons with SCV varies, likely due to varying definitions of “elite control” and biologic variability between persons. For example, Okulicz et al [14] observed that average blood CD4+ T cell levels rose for eight years of infection from ~650 to ~950 cells/µL and then remained stable, inferring that >90% of persons maintain counts above 350 cells/µL for 20 years, while Lambotte et al [8] described more consistent loss with blood CD4+ T cell slopes ranging from −40 to > +12 cells/µL/year. Our data show wide biologic variability between persons, spanning these prior observations, and generally fit the relationship between ongoing viremia and disease progression, with SCV reflecting low ongoing viremia (below the standard limit of detection) associated with slow loss of blood CD4+ T cells. Again, this suggests that persons with SCV are not qualitatively different than those with persistently detectable viremia, but simply reflect a quantitative extreme among infected persons.
In summary, we provide detailed observations of SCV in a diverse cohort of >46,000 persons. The prevalence of SCV varies according to race and gender, and the natural history of untreated SCV may also be affected by these demographics, although larger studies will be required for reliable comparisons. The pattern of viremia “blips” in persons with SCV likely reflects set-point viremia levels below the limit of standard detection assays with biological and/or assay variability that yields occasional values within the detectable range. Persons observed to lose SCV had higher frequencies of these blips, possibly reflecting higher set-point viremia levels (still in the undetectable range) than persons who maintained SCV. These findings support the concept that persons with SCV are not qualitatively distinct from other persons with HIV-1 infection, but rather represent an extreme in the continuum of viremia containment and disease progression.
Supplementary Material
Acknowledgments
OOY conceived the study, analyzed data, and wrote the manuscript. WGC analyzed data and co-wrote the manuscript. RE analyzed data. DL analyzed data. KWC analyzed data and co-wrote the manuscript.
This work was funded by the AIDS Healthcare Foundation; additional support was provided by the UCLA AIDS Institute Center for AIDS Research via the National Institute of Allergy and Infectious Diseases at the Institutes of Health (grant AI028697).
References
- 1.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 2.Hubert JB, Burgard M, Dussaix E, Tamalet C, Deveau C, Le Chenadec J, et al. Natural history of serum HIV-1 RNA levels in 330 patients with a known date of infection. The SEROCO Study Group. AIDS. 2000;14:123–131. doi: 10.1097/00002030-200001280-00007. [DOI] [PubMed] [Google Scholar]
- 3.Walker BD, Yu XG. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol. 2013;13:487–498. doi: 10.1038/nri3478. [DOI] [PubMed] [Google Scholar]
- 4.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 6.Alexander L, Weiskopf E, Greenough TC, Gaddis NC, Auerbach MR, Malim MH, et al. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol. 2000;74:4361–4376. doi: 10.1128/jvi.74.9.4361-4376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckheit RW, 3rd, Allen TG, Alme A, Salgado M, O'Connell KA, Huculak S, et al. Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat Commun. 2012;3:716. doi: 10.1038/ncomms1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005;41:1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 9.Miura T, Brockman MA, Brumme CJ, Brumme ZL, Carlson JM, Pereyra F, et al. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J Virol. 2008;82:8422–8430. doi: 10.1128/JVI.00535-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81:2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2011;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 14.Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. 2009;200:1714–1723. doi: 10.1086/646609. [DOI] [PubMed] [Google Scholar]
- 15.Olson AD, Meyer L, Prins M, Thiebaut R, Gurdasani D, Guiguet M, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS One. 2014;9:e86719. doi: 10.1371/journal.pone.0086719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatano H, Delwart EL, Norris PJ, Lee TH, Dunn-Williams J, Hunt PW, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinoso JB, Kim SY, Siliciano RF, Blankson JN. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis. 2008;47:102–104. doi: 10.1086/588791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabar S, Selinger-Leneman H, Abgrall S, Pialoux G, Weiss L, Costagliola D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS. 2009;23:1163–1169. doi: 10.1097/QAD.0b013e32832b44c8. [DOI] [PubMed] [Google Scholar]
- 21.Okulicz JF, Lambotte O. Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS. 2011;6:163–168. doi: 10.1097/COH.0b013e328344f35e. [DOI] [PubMed] [Google Scholar]
- 22.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 23.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–672. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 24.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344:720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 25.Touloumi G, Pantazis N, Babiker AG, Walker SA, Katsarou O, Karafoulidou A, et al. Differences in HIV RNA levels before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS. 2004;18:1697–1705. doi: 10.1097/01.aids.0000131395.14339.f5. [DOI] [PubMed] [Google Scholar]
- 26.Meditz AL, MaWhinney S, Allshouse A, Feser W, Markowitz M, Little S, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203:442–451. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anastos K, Gange SJ, Lau B, Weiser B, Detels R, Giorgi JV, et al. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr. 2000;24:218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Smith PR, Sarner L, Murphy M, James B, Thomas JM, Skinner CJ, et al. Ethnicity and discordance in plasma HIV-1 RNA viral load and CD4+ lymphocyte count in a cohort of HIV-1-infected individuals. J Clin Virol. 2003;26:101–107. doi: 10.1016/s1386-6532(02)00180-4. [DOI] [PubMed] [Google Scholar]
- 29.Sehgal S, Minz RW, Saikia B, Pasricha N. Slow progressor of human immunodeficiency virus: 20 years follow-up of a case from North India. Indian J Med Microbiol. 2014;32:75–76. doi: 10.4103/0255-0857.124325. [DOI] [PubMed] [Google Scholar]
- 30.Xue X, Sun G, Liu C, Liu J, Tian S, Wang Z. A follow-up study of HIV long-term non-progress populations in Henan province. Zhonghua Yu Fang Yi Xue Za Zhi. 2014;48:684–687. [PubMed] [Google Scholar]
- 31.Murakoshi H, Akahoshi T, Koyanagi M, Chikata T, Naruto T, Maruyama R, et al. Clinical Control of HIV-1 by Cytotoxic T Cells Specific for Multiple Conserved Epitopes. J Virol. 2015;89:5330–5339. doi: 10.1128/JVI.00020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.