Mass spectrum
- 1. Mass Spectrometry Presented By :- Ms. ARTI R RAJPUT M.Pharm, (SUCOP,Pune) 1
- 2. Contents Introduction of Mass spectrum. Types of Ions Molecular ion, Metastable ions, Fragment ions. Fragmentation procedure Fragmentation patterns Fragment characteristics Relative abundances of isotopes. 2
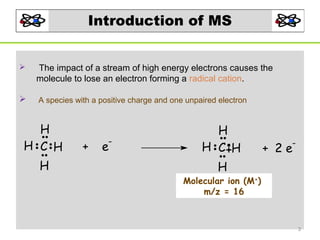
- 3. Introduction of MS The impact of a stream of high energy electrons causes the molecule to lose an electron forming a radical cation. A species with a positive charge and one unpaired electron H H C H H + - e H H C H - + 2e H Molecular ion (M+) m/z = 16 3
- 4. Introduction of MS Only cations are detected. - Radicals are “invisible” in MS The amount of deflection observed depends on the mass to charge ratio (m/z). -Most cations formed have a charge of +1 so the amount of deflection observed is usually dependent on the mass of the ion. . 4
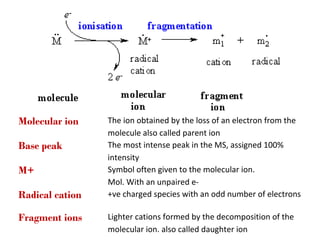
- 5. Molecular ion Base peak M+ Radical cation Fragment ions The ion obtained by the loss of an electron from the molecule also called parent ion The most intense peak in the MS, assigned 100% intensity Symbol often given to the molecular ion. Mol. With an unpaired e+ve charged species with an odd number of electrons Lighter cations formed by the decomposition of the molecular ion. also called daughter ion
- 6. Mass Spectrum The resulting mass spectrum is a graph of the mass of each cation vs. its relative abundance. Relative abundance of an ion means the % of total ion current. Mass spectrum is an analytical techniques which can provide information concerning the molecular structure of organic comp. Base peak is the highest peak or the most intense peak in the spectrum. 6
- 7. Types of Ion Types of ion produced in MS 1.Molecular ions (parent ion) 2.Metastable ions 3.Fragment ions (Dissociation process) 4.Rearrangement ions 5.Multiple charged ions 6.Isotopes ions 7.Negative ions 8.Base peak 7
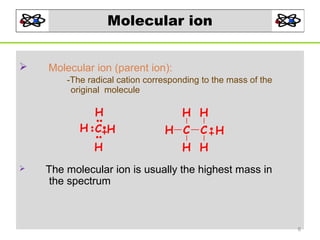
- 8. Molecular ion Molecular ion (parent ion): -The radical cation corresponding to the mass of the original molecule H H C H H H C C H H H H H The molecular ion is usually the highest mass in the spectrum 8
- 9. Molecular ion When a sample sub.is bombarded with electrons of energies of 9 to 15eV, the molecular ion is produced by loss of a single electron. This will give rise to a very simple mass spectrum with essentially all of the ion appearing in one peak called parent peak. M + e = M+ + 2e- Most important ion. 9
- 10. Molecular ion In organic compound there is generally a small peak appearing one mass unit higher than the parent peak (M+1) due to small but observable ,natural abundance of C13 and H2 in these compound. The relative height of parent peak decreases in the following order, aromatic>conjugated olefins>sulphides> unbranched>hydrocarbon>ketones>amine>ester> ethers >carboxylic acid>branched hydrocarbons. 10
- 11. Molecular ion If a molecule yields the parent peak due to molecular ion ,the exact molecular weight can be calculated. Molecular ion are formed in the ground state and in the electronically excited state. 11
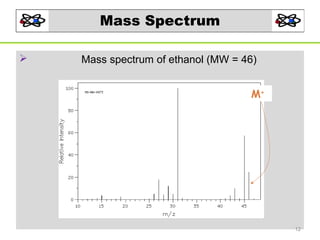
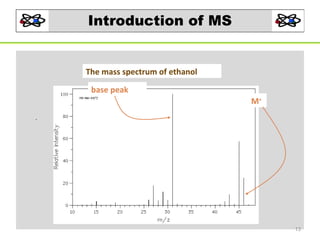
- 12. Mass Spectrum Mass spectrum of ethanol (MW = 46) • Mass spectrum of ethanol (MW = 46) M+ + M 12
- 13. Introduction of MS The mass spectrum of ethanol base peak M+ . 13
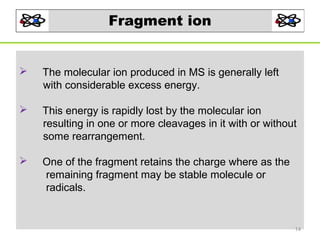
- 14. Fragment ion The molecular ion produced in MS is generally left with considerable excess energy. This energy is rapidly lost by the molecular ion resulting in one or more cleavages in it with or without some rearrangement. One of the fragment retains the charge where as the remaining fragment may be stable molecule or radicals. 14
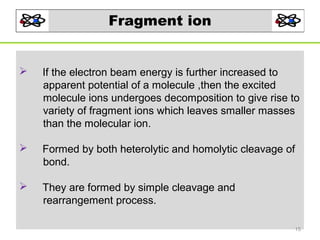
- 15. Fragment ion If the electron beam energy is further increased to apparent potential of a molecule ,then the excited molecule ions undergoes decomposition to give rise to variety of fragment ions which leaves smaller masses than the molecular ion. Formed by both heterolytic and homolytic cleavage of bond. They are formed by simple cleavage and rearrangement process. 15
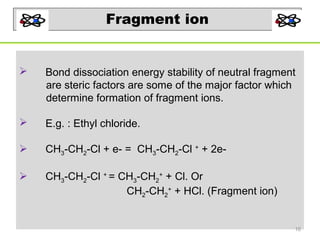
- 16. Fragment ion Bond dissociation energy stability of neutral fragment are steric factors are some of the major factor which determine formation of fragment ions. E.g. : Ethyl chloride. CH3-CH2-Cl + e- = CH3-CH2-Cl + + 2e- CH3-CH2-Cl + = CH3-CH2+ + Cl. Or CH2-CH2+ + HCl. (Fragment ion) 16
- 17. M + e- M+* + 2e- OR +* 1 M + M2 M4+ Fragmentation Process + M3*
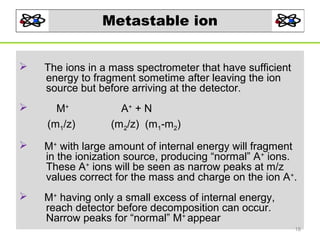
- 18. Metastable ion The ions in a mass spectrometer that have sufficient energy to fragment sometime after leaving the ion source but before arriving at the detector. M+ (m1/z) M+ with large amount of internal energy will fragment in the ionization source, producing “normal” A+ ions. These A+ ions will be seen as narrow peaks at m/z values correct for the mass and charge on the ion A+. M+ having only a small excess of internal energy, reach detector before decomposition can occur. Narrow peaks for “normal” M+ appear A+ + N (m2/z) (m1-m2) 18
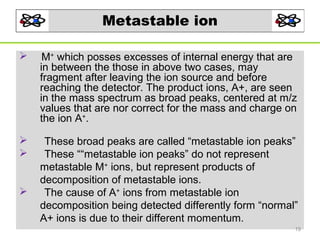
- 19. Metastable ion M+ which posses excesses of internal energy that are in between the those in above two cases, may fragment after leaving the ion source and before reaching the detector. The product ions, A+, are seen in the mass spectrum as broad peaks, centered at m/z values that are nor correct for the mass and charge on the ion A+. These broad peaks are called “metastable ion peaks” These ““metastable ion peaks” do not represent metastable M+ ions, but represent products of decomposition of metastable ions. The cause of A+ ions from metastable ion decomposition being detected differently form “normal” A+ ions is due to their different momentum. 19
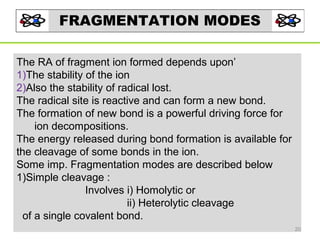
- 20. FRAGMENTATION MODES The RA of fragment ion formed depends upon’ 1)The stability of the ion 2)Also the stability of radical lost. The radical site is reactive and can form a new bond. The formation of new bond is a powerful driving force for ion decompositions. The energy released during bond formation is available for the cleavage of some bonds in the ion. Some imp. Fragmentation modes are described below 1)Simple cleavage : Involves i) Homolytic or ii) Heterolytic cleavage of a single covalent bond. 20
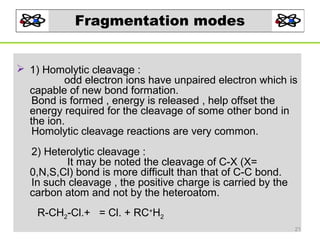
- 21. Fragmentation modes 1) Homolytic cleavage : odd electron ions have unpaired electron which is capable of new bond formation. Bond is formed , energy is released , help offset the energy required for the cleavage of some other bond in the ion. Homolytic cleavage reactions are very common. 2) Heterolytic cleavage : It may be noted the cleavage of C-X (X= 0,N,S,Cl) bond is more difficult than that of C-C bond. In such cleavage , the positive charge is carried by the carbon atom and not by the heteroatom. R-CH2-Cl.+ = Cl. + RC+H2 21
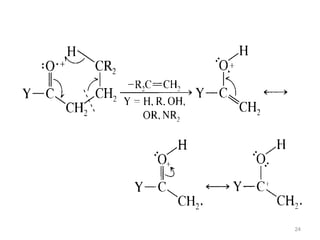
- 22. Fragmentation modes 2) Retro –Diels –Alder reaction The reaction is an example of multicentre fragmentation which is characteristic of cyclic olefins. It involves the cleavage of two bonds of a cyclic system , result the formation of 2 stable unsaturated fragment in which 2 new bonds are formed. This process is not accompanied by any hydrogen transfer rearrangement. The charge can be carried by any one of the fragment. 22
- 23. • • 3)Mc Lafferty Rearrangement: This involves migration of hydrogen atom from one part of the ion to another. To undergo a Mc Lafferty Rearrangement a molecule must possess a) An appropriately located heteroatom e.g. O, N b) A pi electron system ( usually a double bond) & c) An abstractable hydrogen atom gamma to the C = X system Gamma hydrogen atom is transferred through a six membered transition state to an electron deficient centre followed by cleavage at beta bond. The reaction results in the elimination of a neutral molecule. 23
- 24. 24
- 25. 25
- 26. Rules A number of general rules for predicting prominent peak in electron impact spectra are recorded and can be summarized below 1) most compound give molecule ion peak but some do not . Existence of molecular ion peak in the spectrum is dependent on the stability of molecule 2)In case of alkenes , the relative intensity of the molecule ion peak is greatest for the straight chain compound but, a) The intensity decreases with increases degree of branching. b) The intensity decreases with increasing molecular weight in a homologous series. 26
- 27. Rules 3) cleavage is favored at alkyl substituted carbons ,the more substituted ,the more likely is the cleavage .Hence the tertiary carbocation is more suitable than secondary, which is more turn stable then primary. The cation stability order is CH3 < R-CH2 <R2 CH+ < R3C+.Generally the largest substituent at a branch is eliminated most readily as a radical, presumably because a long chain radical can achieve some stability by delocalization of the lone electrons. 4)In alkyl substituted ring compounds, cleavage is favoured at the bound at the bond beta to the ring giving the resonance stabilized benzyl ion. 5)Saturated rings containing side chain, lose the side chains at the alpha bond. the ve+ charge tend to stay with ring fragment. 27
- 28. Rules 6)The cleavage of a C-X bond is more difficult than that of a C-C bond (X=O, N, S, F, CI, etc). If occurred ,the positive charge is carried by the carbon atoms, and not to the heteroatom.the halogens having great electron affinity do not have tendency to carry the positive charge. 7)Double bonds favour allylic cleavage and give the resonance stabilized allylic carbonium ion. 8)Compounds containing a carbonyl group tend to break at this group with positive charge remaining with the carbonyl portions. 28
- 29. Rules 9)During fragmentation, small, suitable neutral molecules e.g. water, carbon monoxide, alcohol, ammonia, hydrogen cyanide, carbon dioxide, ethylene etc, are eliminated from appropriate ions. 29
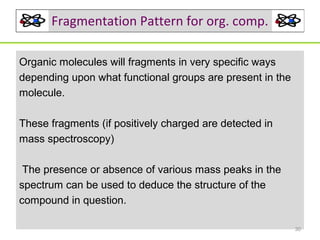
- 30. Fragmentation Pattern for org. comp. Organic molecules will fragments in very specific ways depending upon what functional groups are present in the molecule. These fragments (if positively charged are detected in mass spectroscopy) The presence or absence of various mass peaks in the spectrum can be used to deduce the structure of the compound in question. 30
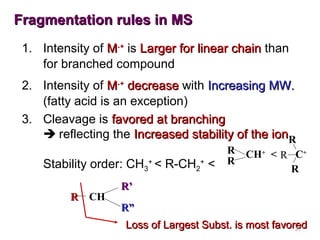
- 31. Fragmentation rules in MS 1. Intensity of M.+ is Larger for linear chain than for branched compound 2. Intensity of M.+ decrease with Increasing MW. (fatty acid is an exception) 3. Cleavage is favored at branching reflecting the Increased stability of the ionR Stability order: CH3+ < R-CH2+ < R CH R R CH+ < R C+ R R’ R” Loss of Largest Subst. is most favored 31
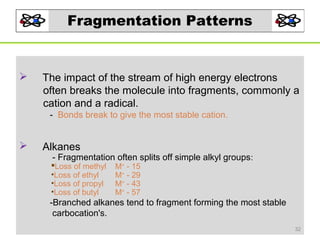
- 32. Fragmentation Patterns The impact of the stream of high energy electrons often breaks the molecule into fragments, commonly a cation and a radical. - Bonds break to give the most stable cation. Alkanes - Fragmentation often splits off simple alkyl groups: Loss of methyl •Loss of ethyl •Loss of propyl •Loss of butyl M+ - 15 M+ - 29 M+ - 43 M+ - 57 -Branched alkanes tend to fragment forming the most stable carbocation's. 32
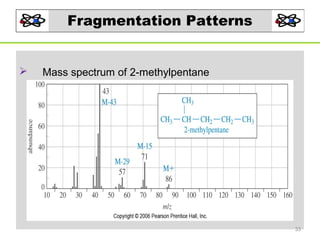
- 33. Fragmentation Patterns Mass spectrum of 2-methylpentane 33
- 34. Fragmentation Patterns Alkenes: -Fragmentation typically forms resonance stabilized allylic carbocation. 34
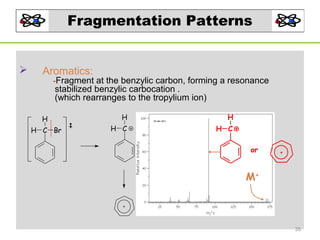
- 35. Fragmentation Patterns Aromatics: -Fragment at the benzylic carbon, forming a resonance stabilized benzylic carbocation . (which rearranges to the tropylium ion) H H C Br H H H C H C or M+ 35
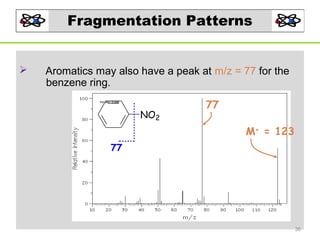
- 36. Fragmentation Patterns Aromatics may also have a peak at m/z = 77 for the benzene ring. NO2 77 M+ = 123 77 36
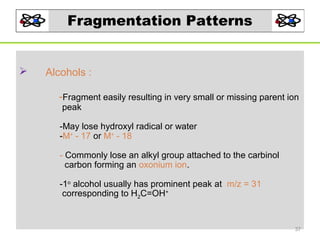
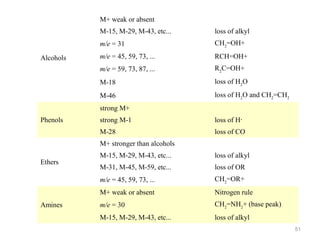
- 37. Fragmentation Patterns Alcohols : -Fragment easily resulting in very small or missing parent ion peak -May lose hydroxyl radical or water -M+ - 17 or M+ - 18 - Commonly lose an alkyl group attached to the carbinol carbon forming an oxonium ion. -1o alcohol usually has prominent peak at m/z = 31 corresponding to H2C=OH+ 37
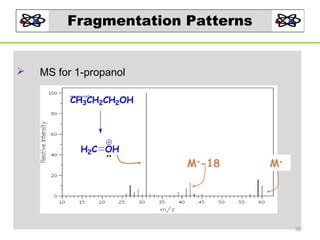
- 38. Fragmentation Patterns MS for 1-propanol CH3CH2CH2OH H2C OH M+-18 M+ 38
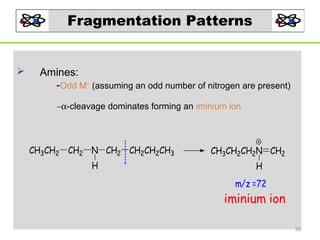
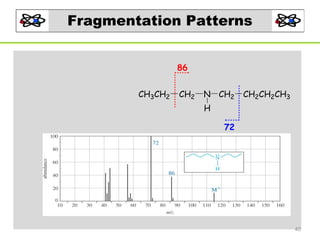
- 39. Fragmentation Patterns Amines: -Odd M+ (assuming an odd number of nitrogen are present) −α-cleavage dominates forming an iminium ion CH3CH2 CH2 N CH2 CH2CH2CH3 H CH3CH2CH2N CH2 H m/z =72 iminium ion 39
- 41. Fragmentation Patterns Ethers - α-cleavage forming oxonium ion - Loss of alkyl group forming oxonium ion - Loss of alkyl group forming a carbocation 41
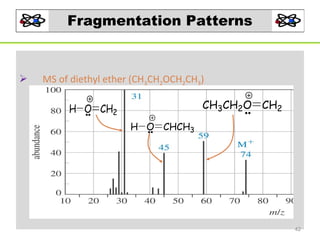
- 42. Fragmentation Patterns MS of diethyl ether (CH3CH2OCH2CH3) CH3CH2O CH2 H O CH2 H O CHCH3 42
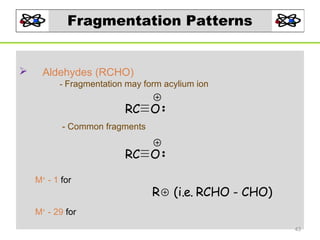
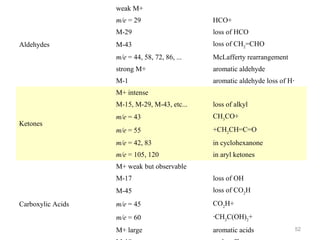
- 43. Fragmentation Patterns Aldehydes (RCHO) - Fragmentation may form acylium ion RC O - Common fragments RC O M+ - 1 for R (i.e. RCHO - CHO) M+ - 29 for 43
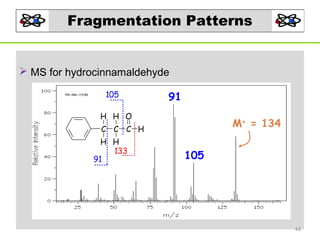
- 44. Fragmentation Patterns MS for hydrocinnamaldehyde 105 91 H H O M+ = 134 C C C H H H 133 91 105 44
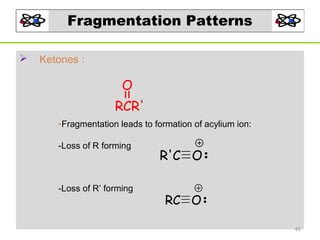
- 45. Fragmentation Patterns Ketones : O RCR' -Fragmentation leads to formation of acylium ion: -Loss of R forming -Loss of R’ forming R'C O RC O 45
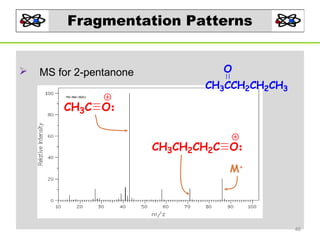
- 46. Fragmentation Patterns MS for 2-pentanone O CH3CCH2CH2CH3 CH3C O CH3CH2CH2C O M+ 46
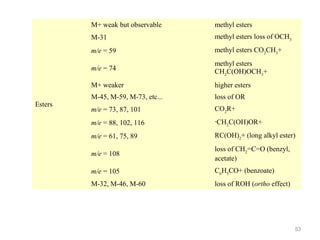
- 47. Fragmentation Patterns Esters (RCO2R’) -Common fragmentation patterns include: Loss of OR’ -peak at M+ - OR’ Loss of R’ -peak at M+ - R’ 47
- 48. Fragmentation Patterns 105 77 O C O CH3 105 77 M+ = 136 48
- 49. • Summary of Fragmentation pattern: 49
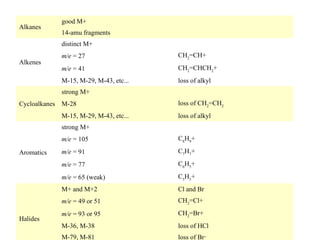
- 50. Alkanes good M+ 14-amu fragments distinct M+ CH2=CH+ m/e = 41 CH2=CHCH2+ M-15, M-29, M-43, etc... Alkenes m/e = 27 loss of alkyl strong M+ Cycloalkanes M-28 M-15, M-29, M-43, etc... loss of CH2=CH2 loss of alkyl strong M+ m/e = 105 C7H7+ C6H5+ m/e = 65 (weak) C5H5+ M+ and M+2 Cl and Br m/e = 49 or 51 Halides m/e = 91 m/e = 77 Aromatics C8H9+ CH2=Cl+ m/e = 93 or 95 CH2=Br+ M-36, M-38 loss of HCl M-79, M-81 loss of Br·
- 51. M+ weak or absent M-15, M-29, M-43, etc... m/e = 31 CH2=OH+ m/e = 45, 59, 73, ... RCH=OH+ m/e = 59, 73, 87, ... R2C=OH+ M-18 loss of H2O M-46 Alcohols loss of alkyl loss of H2O and CH2=CH2 strong M+ Phenols strong M-1 loss of H· M-28 loss of CO M+ stronger than alcohols M-31, M-45, M-59, etc... loss of OR CH2=OR+ M+ weak or absent Amines loss of alkyl m/e = 45, 59, 73, ... Ethers M-15, M-29, M-43, etc... Nitrogen rule m/e = 30 CH2=NH2+ (base peak) M-15, M-29, M-43, etc... loss of alkyl 51
- 52. weak M+ m/e = 29 M-29 loss of HCO M-43 loss of CH2=CHO m/e = 44, 58, 72, 86, ... McLafferty rearrangement strong M+ aromatic aldehyde M-1 Aldehydes HCO+ aromatic aldehyde loss of H· M+ intense M-15, M-29, M-43, etc... m/e = 43 CH3CO+ m/e = 55 +CH2CH=C=O m/e = 42, 83 in cyclohexanone m/e = 105, 120 Ketones loss of alkyl in aryl ketones M+ weak but observable M-17 M-45 Carboxylic Acids loss of OH loss of CO2H m/e = 45 CO2H+ m/e = 60 ·CH2C(OH)2+ M+ large aromatic acids 52
- 53. M+ weak but observable M-31 methyl esters loss of OCH3 m/e = 59 methyl esters CO2CH3+ m/e = 74 methyl esters CH2C(OH)OCH3+ M+ weaker Esters methyl esters higher esters M-45, M-59, M-73, etc... loss of OR m/e = 73, 87, 101 CO2R+ m/e = 88, 102, 116 ·CH2C(OH)OR+ m/e = 61, 75, 89 RC(OH)2+ (long alkyl ester) m/e = 108 loss of CH2=C=O (benzyl, acetate) m/e = 105 C6H5CO+ (benzoate) M-32, M-46, M-60 loss of ROH (ortho effect) 53
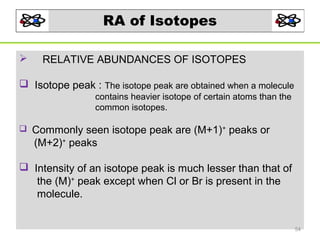
- 54. RA of Isotopes RELATIVE ABUNDANCES OF ISOTOPES Isotope peak : The isotope peak are obtained when a molecule contains heavier isotope of certain atoms than the common isotopes. Commonly seen isotope peak are (M+1)+ peaks or (M+2)+ peaks Intensity of an isotope peak is much lesser than that of the (M)+ peak except when Cl or Br is present in the molecule. 54
- 55. ISOTOPES Most elements occur naturally as a mixture of isotopes. -The presence of significant amounts of heavier isotopes leads to small peaks that have masses that are higher than the parent ion peak. M+1 = a peak that is one mass unit higher than M+ M+2 = a peak that is two mass units higher than M+ 55
- 56. RA of Isotopes RELATIVE ABUNDANCES OF ISOTOPES intensity of an isotope peak depends on the relative abundance of that isotope in nature. The relative abundance of an isotope is calculate on the basis of 100molecules. From RA, the intensity of (M+1)+, (M+2)+ peaks can be determined. For a compound containing one carbon atom , out of every 100 molecules, 98.892 molecule contain C12 isotope and 1.108 molecule contain C13 isotope 56
- 57. RA of Isotopes RELATIVE ABUNDANCES OF ISOTOPES Hence , the intensity of (M+1)+ peak is about 1.1% of the intensity of the (M) +peak and the ratio of the intensities of M+ and (M+1)+ peak is 98.892:1.108. For compound containing silicon , the intensities of (M) + peak corresponding to Si28 isotope , (M+1) + peak corresponding to Si29 isotope and (M+2) + peak corresponding to Si30 isotope are in proportion of their relative abundance in the nature , i.e. 92.18:4.71:3.12. 57
- 58. RA of Isotopes RELATIVE ABUNDANCES OF ISOTOPES For compound containing sulphur , the ratio of intensities of (M) +: (M+2) + peaks , corresponding to S32 and S34 isotopes is 95.018:4.215. The height of the peak is the measure of intensity of that peak. Fluorine and iodine have only one naturally occurring isotope corresponding to atomic mass of 19 and 127, resp. Hence they produced only one peak corresponding to (M) + ion. 58
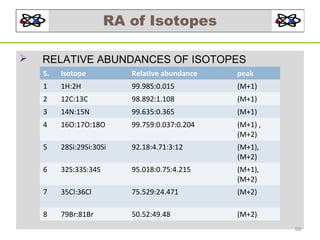
- 59. RA of Isotopes RELATIVE ABUNDANCES OF ISOTOPES S. Isotope Relative abundance peak 1 1H:2H 99.985:0.015 (M+1) 2 12C:13C 98.892:1.108 (M+1) 3 14N:15N 99.635:0.365 (M+1) 4 16O:17O:18O 99.759:0.037:0.204 (M+1) , (M+2) 5 28Si:29Si:30Si 92.18:4.71:3:12 (M+1), (M+2) 6 32S:33S:34S 95.018:0.75:4.215 (M+1), (M+2) 7 35Cl:36Cl 75.529:24.471 (M+2) 8 79Br:81Br 50.52:49.48 (M+2) 59
- 60. References: 1. Silverstein R.M., & Webster F.X, Spectrometric Identification of Organic Compounds, Sixth edition 2006, Page no. 2 – 28. 2. Sharma Y. R. Elementary Organic Spectroscpoy Principles and Chemical Application, Fourth edition 2007, S. Chand & Company, Page no. 280 – 339. 3. Chatwal G.R., Aanand S.K., Instrumental Methods ofAnalysis, Himalaya Publishing House, 5th Edition, Page no. 2.272-2.302 4. http://www.chemistry.ccsu.edu/glagovich/teaching/316/index.ht ml access date - 19 sept 2013 5. http://en.wikipedia.org/wiki/Mass_spectrometry access date – 19 sept 2013 6. Dr. supriya s. mahajan,Instuumental methods of analysis , page no. 125 -154 60
- 61. 61
- 62. 62