Aseptic packaging
- 1. Aseptic Packaging FB- 355 Technological applications in Food Processing
- 2. Why Foods are processed? • Increase Shelf life of product • Availability of commodity throughout year • Wide range of products can be produce • Convenience for consumer
- 3. How Foods products are processed ? • By Application of heat – Aseptic packaging • By Removal of heat • By Irradiation • By Addition of sugar / salt / Additive • Minimal Processing • By Altering surrounding of product
- 4. Aseptic Packaging- • It is defined as the filling of a commercially sterile product into a sterile container under aseptic conditions and hermetically sealing the containers so that reinfection is prevented. • This results in a product which is shelf stable at ambient conditions. • The term aseptic is derived from the Greek word “septicos” which means the absence of putrefactive micro organisms.
- 5. • There are two specific fields of application of aseptic packaging technology- 1. Packaging of pre-sterilised and sterile products. E.g. Milk and dairy products, puddings, desserts, fruit and vegetables. 2. Packaging of non-sterile products to avoid infection by micro-organism E.g. Fermented dairy products like yoghurt.
- 6. • Aseptic packaging technology is fundamentally different from that of conventional food processing by canning. • In canning, the process begins with treating the food prior to filling. • Initial operations inactivate enzymes so that these will not degrade the product during processing. • The package is cleaned, and the product is introduced into the package, usually hot. • Generally, air that can cause oxidative damage is removed from the interior.
- 7. • The package is hermetically sealed and then subjected to heating. • The package must be able to withstand heat up to about 100°C for high acid products and up to 127°C for low acid products. • Figure 1 is a simple illustration comparing the basic difference between conventional canning and aseptic packaging processes for the production of shelf-stable food products.
- 9. Aseptic Processing – Methodology Aseptic processing comprises the following: • Sterilisation of the products before filling. • Sterilisation of packaging materials or containers and closures before filling • Sterilisation of aseptic installations before operation (UHT unit, lines for products, sterile air and gases, filler and relevant machine zones) • Maintaining sterility in this total system during operation
- 10. Sterilization of Products • In aseptic processing, the design to achieve commercial stability is based on the well-founded principles of thermal bacteriology and integrated effect of time/temperature treatment on spores of micro-organisms. • Pre-sterilization of a product usually consists of heating the product to the desired UHT temperature, maintaining this temperature for a given period in order to achieve the desired degree of sterility.
- 11. Some of the latest methods of sterilisation of products include: 1. Microwaves 2. Electrical resistance heating 3. High voltage discharge 4. Ultra high pressure 5. Sterilization of air by incineration
- 12. Sterilisation of Aseptic Packaging Materials and Equipment • Primary packaging Material Tetrapack – LD / Al foil / Paperboard Aseptic Bulk pack – EVOH / Al foil / LD Glass bottle FFS cartons • Secondary packaging Material Tetrapack – pack in corrugated tray with shrink wrapping Aseptic Bulk pack – CFB boxes (25 kg) – Bag in box Aseptic Bulk pack – Plastic Drums (50 kg) Aseptic Bulk pack – MS Drum (225 kg)
- 13. Heat • Systems employing moist heat are frequently sterilized at temperatures ranging from 121°C to 129°C, while 176°C to 232°C is used for sterilization by dry heat. • Sterilization of air by incineration: Temperature ranging from 260°C to 315°C.
- 14. Chemicals • Hydrogen peroxide (at concentrations of 30 to 35%) is the overwhelming choice for use as a chemical sterilant. • Some other chemicals are various acids, ethanol, ethylene oxide and peracetic acid.
- 15. Radiation • Gamma-radiation has been used for decades to decontaminate packaging materials for use in aseptic systems for packing acid and acidified food. • A dose of approximately 1.5 Megaradians (Mrad) is commonly used to decontaminate containers for acid and acidified food. • Ultraviolet (UV-C) light has been used to decontaminate food contact surfaces.
- 16. Types of Aseptic Packs • Carton Boxes, Bags and Pouches, Cups and Trays, Bottles and Jars, Metal Cans, Plastic Cans, Composite Cans are used for packaging.
- 17. Integrity Check for Aseptic Packaging material • Teardown test – Sealing strength • Electrolytic Test – Pinhole testing • Dye test – For pinholes in laminated cartons • Sterility check
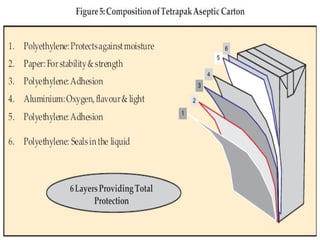
- 18. Composition of Tetra Pak Aseptic Cartons • Tetra Pak aseptic cartons are made of three basic materials that together result in a very efficient, safe and light-weight package. • Each material provides a specific function Figure. • Benefits: Higher degree of safety, hygiene and nutrient retention in food; Preserving taste and freshness; Can be kept for months with no need for refrigeration; Efficient.
- 20. Type of Package Forms available in India • In India, Tetra Pak offers the following packaging systems currently: TBA: Tetra Brik Aseptic TCA: Tetra Classic Aseptic TFA: Tetra Fino Aseptic TWA: Tetra Wedge Aseptic
- 21. Advantages of Aseptic Packaging: Convenience Aseptic packages are portable, lightweight, and shatterproof and easily transportable Food Safety The aseptic process and carton together ensure that the liquid food or beverage inside is free from harmful bacteria and contaminants. No refrigeration required Long shelf life More nutrition Compared with canning, products can retain more nutrients as well as natural taste, colour and texture Low Packaging to product ratio
- 22. Limitations • Plant Installation cost is high as compare to canning • Gas transmission rate of Aseptic bag/ package • Overcooked flavour in some products • Lack of equipment for particulate sterilization, due especially to settling of solids and thus over processing
- 23. Thank you…