Anaesthesia for posterior fossa surgery
- 1. By Dr. Basant Kumar Dindor Under guidance of Dr. C. K. Vyas
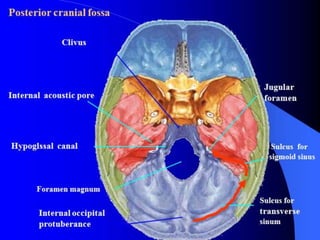
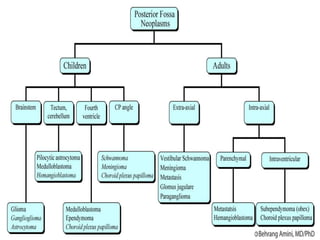
- 2. Posterior Fossa Boundaries : Anteriorly : clivus, petrous part of temporal bone Posteriorly : occipital bone Laterally : squamous and mastoid part of the temporal bone Superiorly : tentorium cerebelli Inferiorly : Foramen magnum Contents : Cerebellar hemispheres, large portion of the brainstem (lower midbrain, pons and upper medulla) 3rd to 12th cranial nerves nuclei and many efferent and afferent fiber tracts that connect the brain with the rest of the body. Blood supply : through vertebrobasilar system ,located mostly anteriorly.
- 4. Clinical physiology Presence of cerebellum ,mid brain, pons, medulla and multiple cranial nerves in posterior cranial fossa provides lesions in this area with a multitude of possible signs and symptoms. Mass effects of lesion ,hydrocephalus secondary to obstruction of CSF flow through aqueduct of sylvius leads to increased ICP .
- 5. Sign and symptoms Initial non specific symptoms include listlessness, headache, fatigue, vomitting, anorexia and personality changes Cerebellar or brainstem signs develop – dysmetria, hemiparesis and cranial nerve deficits. More specific clinical syndromes may occur with tumors that rapidly involve neural structures such as acoustic neuromas, other CP angle tumors, brainstem glioma, carotid body tumor. Sign and symptom due to SOL in posterior fossa are as a result of elevated ICP due to CSF outflow obstruction. In infants an enlarged head or bulging fontanelle may indicate hydrocephalus.
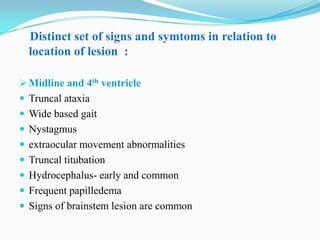
- 6. Distinct set of signs and symtoms in relation to location of lesion : Midline and 4th ventricle Truncal ataxia Wide based gait Nystagmus extraocular movement abnormalities Truncal titubation Hydrocephalus- early and common Frequent papilledema Signs of brainstem lesion are common
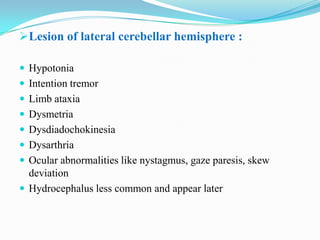
- 7. Lesion of lateral cerebellar hemisphere : Hypotonia Intention tremor Limb ataxia Dysmetria Dysdiadochokinesia Dysarthria Ocular abnormalities like nystagmus, gaze paresis, skew deviation Hydrocephalus less common and appear later
- 8. Herniation of the cerebellar tonsils through foramen magnum (especially in children) appear as : Meningismus Head tilt Muscle spasm Opisthotonus Vomiting Skew deviation of the eyes Downbeat nystagmus (vertical nystagmus) Typical „posturing‟ from tonsillar herniation may be mistaken for “cerebellar fits” Bulbar palsies with vocal cord paralysis
- 9. Swallowing and gag dysfunction Occipital headache Neck pain Coughing may induce paroxysms of increased symptoms including loss of consciousness as the tonsils are further impacted into the foramen magnum Further herniation compresses the medulla irregular respiration and death may result
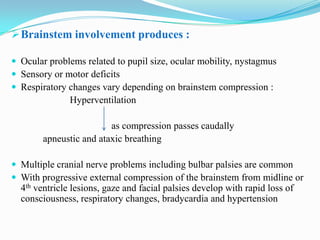
- 10. Brainstem involvement produces : Ocular problems related to pupil size, ocular mobility, nystagmus Sensory or motor deficits Respiratory changes vary depending on brainstem compression : Hyperventilation as compression passes caudally apneustic and ataxic breathing Multiple cranial nerve problems including bulbar palsies are common With progressive external compression of the brainstem from midline or 4th ventricle lesions, gaze and facial palsies develop with rapid loss of consciousness, respiratory changes, bradycardia and hypertension
- 11. Lesion in the posterior fossa may be neoplastic, developmental and vascular processes requiring surgical intervention Success with surgical intervention has become possible because of: Advances in imaging and microsurgical techniques Improved understanding of physiology Advances in perioperative care of the patient Excellent anaesthetic techniques available
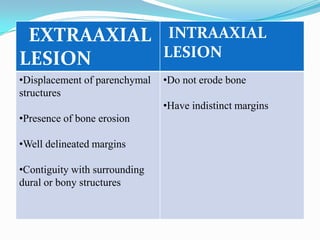
- 13. Preoperative evaluation Bony artefacts are seen in CT scan but MRI scan has greatly improved diagnosis of posterior fossa pathologic conditions Imaging allows intraaxial and extraaxial lesions to be differentiated and allows visualization of the surrounding anatomic structures and they provide information for pathologic diagnosis of lesion.
- 14. EXTRAAXIAL INTRAAXIAL LESION LESION •Displacement of parenchymal •Do not erode bone structures •Have indistinct margins •Presence of bone erosion •Well delineated margins •Contiguity with surrounding dural or bony structures
- 15. Cerebral Angiography : procedure of choice in diagnosing and evaluating aneurysms and vascular malformations Neurophysiologic studies : brainstem auditory evoked responses and electronystagmography are rarely required for diagnosis. But they are of some use for diagnosis of acoustic neurilemmomas and multiple sclerosis Preoperative audiometry is useful in predicting hearing preservation after acoustic shwannoma surgery
- 16. Anaesthetic considerations The posterior fossa is home of brain stem, major motor and sensory pathways cardiovascular and respiratory centers , RAS and lower CN nuclei, thus create challenges to the anaesthesiologists, whose intraoperative goals are to facilitate surgical access, minimise nervous tissue trauma and maintain respiratory and cardiovascular stability.
- 17. 1) Complete Medical History Mainly for function of the heart and the lungs. Most perioperative morbidity and mortality results from poor Cardiac or Pulmonary function. History of CNS Disorders – Seizure disorders need to be assessed for type and for adequacy of therapy. Cerebral hemorrhage or prior strokes are noted. Any residual speech, sensory or motor dysfunction are recorded. Obtain the results of any recent intracranial or diagnostic procedure and consider possibility of residual pneumocephalus.
- 18. 2) Review the patient list of Medications: Steroid, manitol and diuretics, antihypertensive, tricyclic antidepressants ,L –dopa, benzodiazepines, phenothiazines ,which may alter hemodynamics during surgery. 3 ) Physical Examination : • Patient physical status, particularly in reference to cardiovascular and pulmonary stability and airway manageability, is a determinant of the choice of patient position for posterior fossa surgery.
- 19. 4) Neurological Examination : Level of consciousness Document any focal motor or sensory deficit. Examination of sign and symptom of increased ICP. 5) Routine Investigations: CBC Blood Chemistry Coagulation profile ECG and CXR
- 20. Monitoring The goals of monitoring are to ensure adequate CNS perfusion maintain cardiovascular stability and detect and treat VAE ( venous air embolism ) Five lead ECG Pulse oximetry NIBP EtCO2 monitoring Capnography Temperature Precordial stethoscope Central venous catheter Precordial doppler probe Esophageal stethoscope TEE
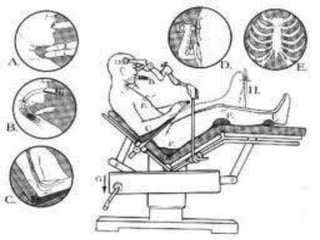
- 21. Patient position Sitting position : Patient head secured in a three pin head holder. Infiltration of the scalp and periosteum at pin site reduces hypertensive response Bony prominences are padded Legs placed in thigh high compression stockings to limit pooling of blood Elbows supported by pillows or pads to avoid contact with table or stretch on brachial plexus and the legs freed of pressure at the level of common peroneal nerve just distal and lateral to the head of fibula Maintain 1 inch space between the chin and chest to prevent cervical cord stretching and obstruction of venous drainage from the face and tongue
- 22. Avoid large airway and bite block placements Avoidance of excessive neck rotation Avoid excessive flexion of knees towards the chest to prevent abdominal compression, lower extremity ischemia and sciatic nerve injury
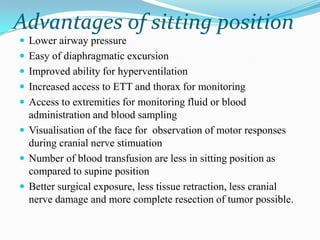
- 24. Advantages of sitting position Lower airway pressure Easy of diaphragmatic excursion Improved ability for hyperventilation Increased access to ETT and thorax for monitoring Access to extremities for monitoring fluid or blood administration and blood sampling Visualisation of the face for observation of motor responses during cranial nerve stimuation Number of blood transfusion are less in sitting position as compared to supine position Better surgical exposure, less tissue retraction, less cranial nerve damage and more complete resection of tumor possible.
- 25. Contraindications for sitting position Intracardiac defects Severe hypovolemia Cachexia Severe hydrocephalus Lesion vascularity extremes of age / impaired cardiac function Degenerative diseases of cervical spine Significant CVD.
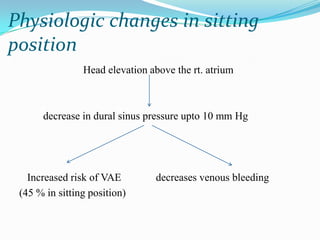
- 26. Physiologic changes in sitting position Head elevation above the rt. atrium decrease in dural sinus pressure upto 10 mm Hg Increased risk of VAE decreases venous bleeding (45 % in sitting position)
- 27. Prone position Patient‟s head elevated to decrease venous bleeding Face compression is prevented by keeping head elevated and shoulders at or above the edge of the operating table Lower incidence of VAE Disadvantages : Surgical field is not as clear as in sitting Eye compression can produce blindness from retinal artery thrombosis Conjunctival edema. Venous pooling in the lower extremities sufficient to impair venous return and hypotension especially in elderly, debilitated patients
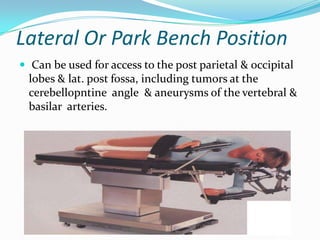
- 29. Lateral Or Park Bench Position Can be used for access to the post parietal & occipital lobes & lat. post fossa, including tumors at the cerebellopntine angle & aneurysms of the vertebral & basilar arteries.
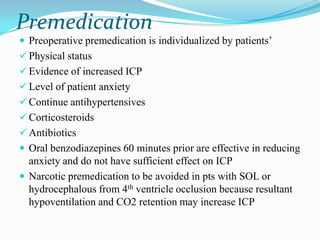
- 30. Premedication Preoperative premedication is individualized by patients‟ Physical status Evidence of increased ICP Level of patient anxiety Continue antihypertensives Corticosteroids Antibiotics Oral benzodiazepines 60 minutes prior are effective in reducing anxiety and do not have sufficient effect on ICP Narcotic premedication to be avoided in pts with SOL or hydrocephalous from 4th ventricle occlusion because resultant hypoventilation and CO2 retention may increase ICP
- 31. Induction It can be performed by various agent. The best induction agent is Barbiturates because its administration provides a profound reduction in CMRO2 , CBF and ICP. Smooth and gentle induction of general anaesthesia is more important. An acceptable induction sequence combines four steps – 1.) Preoxygenation and self hyperventilation 2.) Thiopentone 3-4 mg/kg IV followed by mask ventilation to assure airway patency. 3.) Vecuronium 0.1 mg/kg IV and mask hyperventilation with oxygen and N2O (50:50) until neuromuscular blockade achieved. If there is C/I to use of N2O, add small concentration of isoflurane to the O2. 4.) Lidocaine 1.5 mg/kg IV and additional thiopentone 2 mg/kg IV just before ET Intubation.
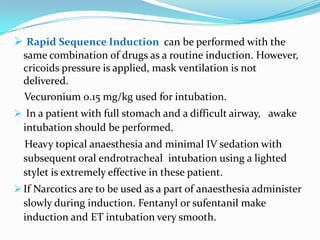
- 32. Rapid Sequence Induction can be performed with the same combination of drugs as a routine induction. However, cricoids pressure is applied, mask ventilation is not delivered. Vecuronium 0.15 mg/kg used for intubation. In a patient with full stomach and a difficult airway, awake intubation should be performed. Heavy topical anaesthesia and minimal IV sedation with subsequent oral endrotracheal intubation using a lighted stylet is extremely effective in these patient. If Narcotics are to be used as a part of anaesthesia administer slowly during induction. Fentanyl or sufentanil make induction and ET intubation very smooth.
- 33. Maintenance of Anaesthesia It can be accomplished in a number of ways. These technique generally fall in to two categories – 1.) Primarily Volatile agent and 2.) Narcotics Either technique can be used. In Narcotics based anaesthetic technique with either N2O or low dose (< 1%) isoflurane in O2 is optimum . Fentanyl or sufentanil may be used.
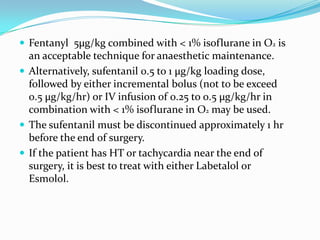
- 34. Fentanyl 5µg/kg combined with < 1% isoflurane in O2 is an acceptable technique for anaesthetic maintenance. Alternatively, sufentanil 0.5 to 1 µg/kg loading dose, followed by either incremental bolus (not to be exceed 0.5 µg/kg/hr) or IV infusion of 0.25 to 0.5 µg/kg/hr in combination with < 1% isoflurane in O2 may be used. The sufentanil must be discontinued approximately 1 hr before the end of surgery. If the patient has HT or tachycardia near the end of surgery, it is best to treat with either Labetalol or Esmolol.
- 35. A volatile agent preferably isoflurane with little or no narcotic supplementation can also be used. Hyperventilation combination with < 1% isoflurane generally results in stable intracranial dynamics. N2O may be used in anaesthetic regimen but it is contraindicated if the patient is suspected to have pneumocephalus (recent intracranial surgery or trauma) or if there is potential for air embolism N2O expand both the pneumocephalus and the air embolus.
- 36. Muscle relaxation is also important during neurosurgery relaxation prevent patient movement at inappropriate time, it may decrease ICP by relaxing the chest wall with decrease intrathoracic pressure and encourage venous drainage.
- 37. Fluid Management A balanced salt solution is the fluid of choice for neurosurgical procedures. The volume of fluid administered should be minimized during the induction of anaesthesia and then kept as low as hemodynamic stability and urine output will allow. Use no dextrose containing solution. Maintain hematocrit at 30 to 35 %. Patients who present for tumor surgery should be kept on the dry side of normal. Excess fluid administered to these patients may cause brain edema at the sites of blood brain barrier disruption. Thus a dry but stable patient is optimum for tumor surgery.
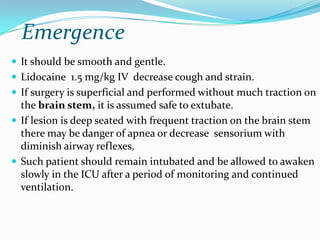
- 38. Emergence It should be smooth and gentle. Lidocaine 1.5 mg/kg IV decrease cough and strain. If surgery is superficial and performed without much traction on the brain stem, it is assumed safe to extubate. If lesion is deep seated with frequent traction on the brain stem there may be danger of apnea or decrease sensorium with diminish airway reflexes, Such patient should remain intubated and be allowed to awaken slowly in the ICU after a period of monitoring and continued ventilation.
- 39. COMPLICATIONS
- 40. 1.Brainstem & CN stimulation Hypertension results from stimulation of Vth CN, periventricular gray area, reticular formation, or nucleus of tractus solitarius. Bradycardia and escape rhythms results from vagus N stimulation, Hypotension can results from pontine or medullary compression. Ventricular and supraventricula arrhythmias can occur from brain stem stimulation. Close attention to cardiovascular parameters during critical periods of surgery is essential and surgeon may inform of brainstem encroachment
- 41. 2.Venous Air Embolism Most Feared Complication associated with sitting position. CAUSES: open veins & non collapsible venous channels gravitational effects of low CVP neg. I.v. pressure relative to atm. Pressure poor surgical technique Incidence- 25-50%
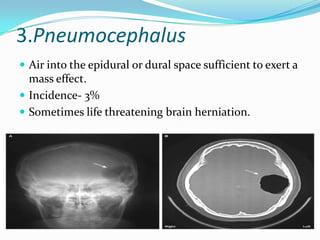
- 42. 3.Pneumocephalus Air into the epidural or dural space sufficient to exert a mass effect. Incidence- 3% Sometimes life threatening brain herniation.
- 43. CAUSES: Diminition of brain volume secondary to mannitol hyperventillation removal of SOL contraction of intravascular blood vol. associated with acute hemorrhage Gravitational effect of sitting position Intraop drainage of CSF “Inverted Pop Bottle Analogy” as CSF pours out, air bubbles to the top of the container(cranium) So that is why slow cont. gravitational drainage of CSF in sitting position can result in accumulation of air in subdural space
- 44. ROLE OF NITROUS OXIDE: major contributing factor avoidance would not eliminate the risk it increases the size of air filled space S/S: Confusion Headache Convulsions Neurological deficits Failure to regain conciousness CT scan confirms the diagnosis and localisation of intracranial air, if untreated Brain herniation and death. T/T: IMMEDIATE twist drill aspiration of air through burr holes on either side of the vertex.
- 45. 4.MACROGLOSSIA CAUSES: Extreme flexion of head with chin resting on the chest Prolong presence of an oral airway Obstruction of its venous and lymphatic drainage Airway obstruction hypoxemia hypercapnia postop
- 46. 4.QUADRIPLEGIA CAUSES: Flexion of head on the neck causes streching of the spinal cord at C5 level, regional cord perfusion may be compromised if MAP is decreased.
- 47. References Manual of NeuroAnaesthesia Scott L. Mears, M.D., and Richard j. Sperry, M.D.
- 48. Thanks