Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
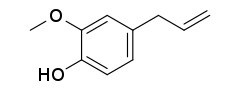
Eugenol /ˈjuːdʒɪnɒl/ is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds.[2] It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf.[3][4][5][6] It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil.[7] Eugenol has a pleasant, spicy, clove-like scent.[8] The name is derived from Eugenia caryophyllata, the former Linnean nomenclature term for cloves. The currently accepted name is Syzygium aromaticum.[9]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methoxy-4-(prop-2-en-1-yl)phenol | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 1366759 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.355 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Density | 1.06 g/cm3 |
| Melting point | −7.5 °C (18.5 °F; 265.6 K) |
| Boiling point | 254 °C (489 °F; 527 K) |
| Acidity (pKa) | 10.19 at 25 °C |
| −1.021×10−4 cm3/mol | |
| Viscosity |
|
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 104 °C (219 °F; 377 K) |
| Related compounds | |
Related compounds |
2-Phenethyl propionate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The biosynthesis of eugenol begins with the amino acid tyrosine. L-tyrosine is converted to p-coumaric acid by the enzyme tyrosine ammonia lyase (TAL).[10] From here, p-coumaric acid is converted to caffeic acid by p-coumarate 3-hydroxylase using oxygen and NADPH. S-Adenosyl methionine (SAM) is then used to methylate caffeic acid, forming ferulic acid, which is in turn converted to feruloyl-CoA by the enzyme 4-hydroxycinnamoyl-CoA ligase (4CL).[11] Next, feruloyl-CoA is reduced to coniferaldehyde by cinnamoyl-CoA reductase (CCR). Coniferaldeyhyde is then further reduced to coniferyl alcohol by cinnamyl-alcohol dehydrogenase (CAD) or sinapyl-alcohol dehydrogenase (SAD). Coniferyl alcohol is then converted to an ester in the presence of the substrate CH3COSCoA, forming coniferyl acetate. Finally, coniferyl acetate is converted to eugenol via the enzyme eugenol synthase 1 and the use of NADPH.[citation needed]
Eugenol is a metabolite of Caleicine, the active compound found in Calea Ternifolia, and is thought to cause the sedative and hallucinogenic state Calea Ternifolia can induce.

Eugenol and thymol possess general anesthetic properties. Like many other anesthetic agents, these 2-alkyl(oxy)phenols act as positive allosteric modulators of the GABAA receptor. Although eugenol and thymol are too toxic and not potent enough to be used clinically, these findings led to the development of 2-substituted phenol anesthetic drugs, including propanidid (later withdrawn) and the widely used propofol.[12] Eugenol and the structurally similar myristicin, have the common property of inhibiting MAO-A and MAO-B in vitro.[13][14]
Eugenol acts on the NMDA receptors as an antagonist, as well as the histamine receptors as an antagonist, but the exact specifics of this are unknown. [15]
In humans, complete excretion occurs within 24 hour and metabolites are mostly conjugates of eugenol.[16]
Eugenol is used as a flavor or aroma ingredient in teas, meats, cakes, perfumes, cosmetics, flavorings, and essential oils.[2][17][18] It is also used as a local antiseptic and anaesthetic.[19][20] Eugenol can be combined with zinc oxide to form zinc oxide eugenol which has restorative and prosthodontic applications in dentistry. For persons with a dry socket as a complication of tooth extraction, packing the dry socket with a eugenol-zinc oxide paste on iodoform gauze is effective for reducing acute pain.[21] Eugenol-zinc oxide paste is also used for root canal sealing.[22]
It is attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.[23] It also attracts female cucumber beetles.[24]
Eugenol and isoeugenol, which both are floral volatile scent compounds, are catalyzed by a single type of enzyme in the genus Gymnadenia and the gene encoding for this enzyme is the first functionally characterized gene in these species.[25] Eugenol is an ingredient in some insecticides.[2]
Clove oil is common as an anesthetic for use on aquarium fish as well as on wild fish when sampled for research and management purposes.[26][27] Where readily available, it presents a humane method to euthanize sick and diseased fish either by direct overdose or to induce sleep before an overdose of eugenol.[28][29]
Eugenol is an ingredient in some fungicides and weed control products used in agricultural practices in the European Union.[2] It is used in hundreds of household products, such as pesticides, pet care, laundry, cleaning, and paper or vehicle products.[2]
Lesser side effects of eugenol toxicity that may not be considered a full overdose: Sedation, dizziness, hallucinations, mild respiratory depression, nausea, and muscle spasms.
Taken orally in high doses for chronic periods, eugenol may cause liver toxicity.[17] An overdose is possible, causing a wide range of symptoms, such as hematuria (blood in urine), convulsions, diarrhea, delirium, unconsciousness, heavy respiratory depression, tachycardia (rapid heart rate), or acute kidney injury.[17][30][31] N-acetylcysteine may be used to treat people with eugenol or clove oil overdose.[32]
Eugenol is subject to restrictions on its use in perfumery,[33] as some people may become sensitised to it, however, the degree to which eugenol can cause an allergic reaction in humans is disputed.[34]
Eugenol is a component of balsam of Peru, to which some people are allergic.[35][36] When eugenol is used in dental preparations such as surgical pastes, dental packing, and dental cement, it may cause contact stomatitis and allergic cheilitis.[35] The allergy can be discovered via a patch test.[35]
Eugenol naturally occurs in numerous plants, including the following:
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.