L-DOPA
Chemical compound From Wikipedia, the free encyclopedia
l-DOPA, also known as l-3,4-dihydroxyphenylalanine and used medically as levodopa, is made and used as part of the normal biology of some plants[2] and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine.
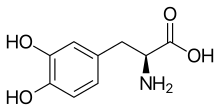 Skeletal formula of L-DOPA | |
 | |
| Names | |
|---|---|
| IUPAC name
(S)-2-Amino-3-(3,4-dihydroxyphenyl)propanoic acid | |
| Other names
l-3,4-Dihydroxyphenylalanine; Levodopa | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.405 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H11NO4 | |
| Molar mass | 197.19 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS.[3][4] In some plant families (of the order Caryophyllales), l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains.[5]
l-DOPA can be manufactured and in its pure form is sold as a drug with the INN levodopa. Trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the treatment of Parkinson's disease and dopamine-responsive dystonia, as well as restless leg syndrome.[6]
l-DOPA has a counterpart with opposite chirality, d-DOPA. As is true for many molecules, the human body produces only one of these isomers (the l-DOPA form). The enantiomeric purity of l-DOPA may be analyzed by determination of the optical rotation or by chiral thin-layer chromatography.[7]
Biological role
Summarize
Perspective
l-DOPA is produced from the amino acid l-tyrosine by the enzyme tyrosine hydroxylase. l-DOPA can act as an l-tyrosine mimetic and be incorporated into proteins by mammalian cells in place of l-tyrosine, generating protease-resistant and aggregate-prone proteins in vitro and may contribute to neurotoxicity with chronic l-DOPA administration.[11] It is also the precursor for the monoamine or catecholamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline). Dopamine is formed by the decarboxylation of l-DOPA by aromatic l-amino acid decarboxylase (AADC).
l-DOPA can be directly metabolized by catechol-O-methyl transferase to 3-O-methyldopa, and then further to vanillactic acid. This metabolic pathway is nonexistent in the healthy body, but becomes important after peripheral l-DOPA administration in patients with Parkinson's disease or in the rare cases of patients with AADC enzyme deficiency.[12]
l-Phenylalanine, l-tyrosine, and l-DOPA are all precursors to the biological pigment melanin. The enzyme tyrosinase catalyzes the oxidation of l-DOPA to the reactive intermediate dopaquinone, which reacts further, eventually leading to melanin oligomers. In addition, tyrosinase can convert tyrosine directly to l-DOPA in the presence of a reducing agent such as ascorbic acid.[13]
History
l-dopa was first islolated from the seeds of the Vicia faba or broad bean plant in 1913 by Swiss biochemist Markus Guggenheim.[14]
The 2001 Nobel Prize in Chemistry was also related to l-DOPA: the Nobel Committee awarded one-quarter of the prize to William S. Knowles for his work on chirally catalysed hydrogenation reactions, the most noted example of which was used for the synthesis of l-DOPA.[15][16][17]
Other organisms
Marine adhesion
l-DOPA is a key compound in the formation of marine adhesive proteins, such as those found in mussels.[18][19] It is believed to be responsible for the water-resistance and rapid curing abilities of these proteins. l-DOPA may also be used to prevent surfaces from fouling by bonding antifouling polymers to a susceptible substrate.[20] The versatile chemistry of l-DOPA can be exploited in nanotechnology.[21] For example, DOPA-containing self-assembling peptides were found to form functional nanostructures, adhesives and gels.[22][23][24][25]
Plants and in the environment
In plants, L-DOPA functions as an allelochemical which inhibits the growth of certain species, and is produced and secreted by a few legume species such as the broad bean Vicia faba and the velvet bean Mucuna pruriens.[26] Its effect is strongly dependent on the pH and the reactivity of iron in the soil.[27] L-DOPA can also be found in cephalopod ink.[28]
Use as a medication and supplement
L-DOPA is used medically under the name levodopa in the treatment of Parkinson's disease and certain other medical conditions. It is usually used in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor such as carbidopa or benserazide. These agents increase the strength and duration of levodopa. Combination formulations include levodopa/carbidopa and levodopa/benserazide, as well as levodopa/carbidopa/entacapone.
L-DOPA is found in high amounts in Mucuna pruriens (velvet bean) and is available and used over-the-counter as a supplement.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.


