Abstract
In human exhaled breath, more than 3000 volatile organic compounds (VOCs) are found, which are directly or indirectly related to internal biochemical processes in the body. Electronic noses (E-noses) could play a potential role in screening/analyzing various respiratory and systemic diseases by studying breath signatures. An E-nose integrates a sensor array and an artificial neural network that responds to specific patterns of VOCs, and thus can act as a non-invasive technology for disease monitoring. The gold standard blood glucose monitoring test for diabetes diagnostics is invasive and highly uncomfortable. This contributes to the massive need for technologies which are non-invasive and can be used as an alternative to blood measurements for glucose detection. While lung cancer is one of the deadliest cancers with the highest death rate and an extremely high yearly global burden, the conventional diagnosis means, such as sputum cytology, chest radiography, or computed tomography, do not support wide-range population screening. A few standard non-invasive techniques, such as mass spectrometry and gas chromatography, are expensive, non-portable, and require skilled personnel for operation and are again not suitable for large-scale screening. Breath contains markers for both diabetes and lung cancer along with markers for several diseases and thus, a non-invasive technique such as the E-nose would greatly improve analysis procedures over existing invasive methods. This review shows the state-of-the-art technologies for VOC detection and machine learning approaches for two clinical models: diabetes and lung cancer detection.
Export citation and abstract BibTeX RIS
1. Introduction
In the diseased state, the biochemical processes of organs are altered, which results in the production of new chemicals or altered consumption of existing chemicals [1, 2]. These abnormal changes alter the composition of the body fluids, resulting in complex gas mixtures of volatile organic compounds (VOCs) in the exhaled breath [3]. These breath signatures can serve as biomarkers for specific diseases and can be a potential candidate for early disease detection as well as analysis of diseases [4, 5]. Non-communicable diseases (NCDs) account for 71% of global deaths annually according to a recent report by the World Health Organization (WHO) [6]. Cardiovascular diseases, cancer, respiratory diseases, and diabetes together account for most of the NCD deaths. Within this set, lung cancer and diabetes mellitus were ranked in the 6th and 7th positions, respectively, in a global list of the top ten diseases causing death in 2016 [7]. These two diseases can be uniquely studied and characterized using the E-nose technology discussed in this review article. Around 422 million people suffered from diabetes (1.6 million deaths) in 2014 and the percentage has been rising over the years [8]. The number of diabetes-related issues, such as renal failure, obesity, visual impairment, and blindness, are increasing day-by-day. Diabetic patients are more susceptible to contracting other diseases compared to a healthy person. Currently, the fasting plasma glucose (FPG) test and oral glucose tolerance test (OGTT) of pricked blood samples are the prominent methods used to diagnose diabetes [9, 10]. These methods are relatively simple, cost-effective, and require only a microliter of blood; however, sometimes they become a painful, physiological burden and discourage patients from screening procedures, especially those patients who need multiple checks in a day.
While diabetes is a slow killer, cancer is more direct. Amongst various types of cancer, lung cancer accounted for the maximum number of deaths in 2016 [11]. Traditionally, lung cancer is diagnosed through a physical examination of blood, urine samples, and atypical physiology detected through techniques such as x-rays, computed tomography (CT), fibre-optic endoscopy, magnetic resonance imaging (MRI), and ultrasound [12]. Most of these techniques are expensive, invasive, time-consuming, or require trained personnel. This affects the coverage and viability of large-scale screening, thereby highlighting the need for a low-cost, non-invasive, point-of-care, and user-friendly diagnostic technique. An electronic nose (E-nose), which consists of an array of gas/VOC sensors integrated with artificial neural networks, has been shown as a promising non-invasive technology for detecting and delineating targeted VOCs from the exhaled breath, resulting in early diagnosis of several diseases [13–19]. This article reviews some of the existing E-nose technologies that have been developed in the last 25 years and their applications towards healthcare, with special focus on diabetes and lung cancer.
2. Electronic nose technology
The human nose and brain are interlinked and trained to detect and distinguish several chemicals, which in turn helps to provide necessary information, such as the freshness of food, pungent smells, perfumes, and gas leakage [20, 21]. Complex volatile compounds interact with neuron cell receptors and signals are then sent through nerve impulses to the brain for pre-processing. In the final step, signals are stored and analyzed with the help of the hypothalamus and olfactory cortex in the brain. In a similar way, E-nose technology has a sensor array, data acquisition system, signal processing unit, data storage facility, and artificial intelligence for detection and analysis of various chemicals in vapour form [22, 23]. A pictorial comparison of the E-nose and mammalian smelling system is shown in figure 1.
Figure 1. A schematic diagram of the mammalian nose and E-nose.
Download figure:
Standard image High-resolution imageSeveral types of chemical sensors based on electrical, thermal, mass, and optical transduction principles, developed up until 1980, although available as a technology, were not able to mimic the mammalian nose. This was due to the unavailability of advanced electronics components and efficient computational tools. The first E-nose was developed using an array of chemical sensors and a pattern recognition system to discriminate gases by Persaud and Dodd in 1982 at Warwick University, UK [24]. Further similar work was extended by Ikegami et al at Hitachi Research Laboratory, Japan [25]. In 1994, Gardner and Bartlett defined the E-nose as 'an instrument which comprises an array of electronic chemical sensors with partial sensitivity and an appropriate pattern recognition system capable of recognizing simple or complex odors' [26]. Over the last decade, researchers working on the E-nose have focused on developing highly sensitive and selective sensors, and a compact electronic module (electronic circuits for amplifying signals, signal conditioning, and analog-to-digital (A/D) converters) for better feature extraction with reduced redundancy as well as classifiers (primarily neural networks) for learning and validation processes, such as a pattern recognition algorithm, to discriminate the chemical of interest. Several E-noses have been developed by combining nonspecific sensor arrays and machine learning (ML) for different applications, such as biomedical, healthcare, safety, food industry, chemical industry, and pharmaceutical. Some of the commercially available E-nose systems are listed in table 1.
Table 1. A list of commercially available E-nose systems on the market.
| Sl. No | Model of E-nose | Company | Sensor type/methods | Number of sensors | Applications | References |
|---|---|---|---|---|---|---|
| 1 | Cyrano 320 | Sensigent, USA | Polymer composite | 32 | Medical, food, industry | [27] |
| 2 | Fox 4000/3000/2000 | Alpha MOS, Toulouse, France | MOS | 18, 12 and 6 | Food, packaging, pharmaceutical | [28] |
| 3 | i-pen, PEN2, PEN3 | Airsense Analytical, Schwerin, Germany | MOS | 6 | Food, industry, safety | [29] |
| 4 | MOSES II | GSG-Analytical, Brushal, Germany | MOS, QCM, GC, GC-MS | 8–40 | Food, industry | [30] |
| 5 | FAIMS technology | Owlstone Medical, Cambridge, UK | IMS | 1 | Medical | [31] |
| 6 | NST3320 | Nordic Sensor Technologies, Sweden | FET, MOS | 32 | Food, air quality | [32] |
| 7 | E-nose Mk3.3, Mk4 | Eveleigh, NSW, Australia | Not mentioned | 4–6 | Air quality, medical, safety | [33] |
| 8 | Aromascan A32S | Osmetech Plc, USA | Composite polymer | 32 | Food, air quality | [34] |
| 9 | EOS 835, Ambiente | Sacmi, Italy | MOS | 6 | Industry | [35] |
MOS: metal oxide semiconductor, QCM: quartz crystal microbalance, GC-MS: gas chromatography-mass spectroscopy, IMS: ion mobility spectrometry, FET: field effect transistor.
2.1. Sensor technologies in the E-nose
Gas sensors are one of the basic elements in the E-nose tool. The ideal sensor needed for an E-nose should have features such as high sensitivity, specificity, stability, reproducibility, short response/recovery time, low sensitivity towards humidity, low operating temperature, small size, low cost, easy calibration, and easily processable output data. In the last fifty years, several sensor types, such as the optical gas sensor, polymers, metal oxide semiconductors (MOS), field effect transistors (FET), catalytic, electrochemical, and piezoelectric sensors, have been used (table 2) [36–40]. Researchers have focused on the sensing principle as well as fabrication techniques to achieve highly sensitive, low cost, easily operable gas sensors, which can detect a wide range of gases over a range of concentrations; for example, a gas sensor can prove to be an efficient tool in an E-nose system.
In an optical transduction gas sensor, the change in absorption properties, luminescence, scattering, refractive index, optical path length, and reflectivity have been used to detect gases. Optical absorption and luminescence-based sensors measure the change in intensity and/or wavelength of light after exposure to gases [41, 42]. Surface plasmon resonance is one of the efficient techniques used for gas sensing application [43]. Although, optical gas sensors are excellent in terms of gas sensitivity, selectivity, cost-effectiveness due to their size, and the complexity of the signal conditioning systems for these sensors, they are not considered as ideal candidates to be used in fabricating an E-nose. The surface acoustic wave (SAW) device and the quartz crystal microbalance (QCM) are two important piezoelectric gas sensors used in some existing E-nose systems. In the QCM, a gas sensitive polymer or semiconducting metal oxide is coated on a piezoelectric disk and the change in the resonant frequency of the traveling wave is measured. [44, 45]. Similarly, the SAW gas sensor measures the gas induced change in the propagation frequency obtained through the piezoelectric substrate coated with sensing material [46, 47]. The advantages of piezoelectric gas sensors are high sensitivity, small size, fast response time, low power consumption, and robustness. However, these piezoelectric sensors have poor signal-to-noise ratio as they operate at very high frequencies and require complex electronic circuits to delineate the signal response, making it difficult to act as a supportive element for an efficient E-nose system.
Polymer composites with different reversible physicochemical properties are used in commercial E-nose systems due to their high sensitivity, selectivity, and capability to operate at room temperature [36]. The physisorption of VOCs is more efficient on a polymer's surface because of dipole/dipole interactions of polymers and VOCs [36, 48]. Most gas sensitive polymers are classified into two major groups: (1) conducting polymers, and (2) non-conducting polymers. The conducting polymer sensor architecture is similar to the MOS gas sensor (changes in conductivity due to gas absorption), whereas non-conducting polymers are generally used in piezoelectric sensors. However, the response/recovery time and durability are issues.
MOS-based gas sensors are considered as one of the most attractive classes of the sensing device [49]. Solid-state resistive metal oxide gas sensors are attractive because of their simplicity, high sensitivity, low cost, stability, and compatibility with modern electronic devices. Several semiconducting metal oxides such as SnO2, ZnO, CuO, TiO2, WO3, and NiO have been used as sensing elements to detect several gases and VOCs. [37, 50–56]. The resistance of these semiconducting metal oxides changes during interaction with analytes. Most of the metal oxide-based sensors operate at a high operating temperature (100 °C–500 °C) and thus, microheaters are used to heat the sensing element locally and to save power [57]. To further reduce the power required to heat the sensing element, the sensors are fabricated on the diaphragm using micro-electro-mechanical system (MEMS)-based technologies [57–59]. A schematic and photograph of a MEMS-based gas sensor using nanostructured metal oxide (ZnO and CuO) is depicted in figures 2(a) and (b) [59]. Over the last decade, several approaches have been used to synthesize nanostructures of metal oxides, and these nanostructures have been used as a sensing element due to their enhanced sensing performance (higher surface-to-volume ratio) [54, 55, 60, 61]. In spite of the many advantages of the MOS sensor, it is observed that the resistive metal oxide gas sensors suffer from poor selectivity. However, using an MOS sensor in an array and integrating with ML algorithms improves selectivity [62]. To enhance the compatibility with the complementary metal oxide semiconductor (CMOS) technologies, FET gas sensors have been studied by several groups [63–66]. In an FET gas sensor, the interaction between the chemical and source-drain channel modifies the electrical behaviour (IV characteristic), such as a shift in the source-drain current, threshold voltage, and carrier mobility. Most common FET gas sensors are nanomaterial-based sensors, where carbon nanotubes (CNTs), graphene, metal oxide nanowires (NWs), Si NWs, metal oxide NWs, and transition metal chalcogenides are used as sensing elements [38, 63, 64, 67]. FET gas sensors are more compatible with standard integrated circuit (IC) fabrication technology compared to other types of gas sensor, and are an ideal choice for fabricating the next-generation E-nose.
Figure 2. (a) A schematic diagram and (b) a photographic image of a MEMS-based gas sensor. Reprinted from [59], Copyright (2016), with permission from Elsevier.
Download figure:
Standard image High-resolution imageTable 2. A comparison of different types of gas detectors.
| Sl. No | Type of gas sensor/instrument (with schematic) | Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| 1 | Semiconductor 
|
Change in resistance or conductivity | Low cost; short response time; long-lasting | Relatively low sensitivity; high energy consumption | [37, 50–52, 54, 55] |
| 2 | Field Effect Transistor (FET) 
|
Change in electrical behavior | Ultra-sensitive; great adsorptive capacity; miniaturized; stability | Difficulties in fabrication; repeatability; high cost | [38, 63, 65, 68] |
| 3 | Polymer 
|
Change in resistance, mass, optical properties | Low cost of fabrication; portable structure; low power consumption | Long response/recovery time; instability; irreversibility; poor selectivity | [36, 48] |
| 4 | Optical 
|
Change in surface plasmon resonance, fluorescence, luminescence, absorbance | High sensitivity; long lifetime | Difficulty in miniaturization; expensive | [39, 43, 69, 70] |
| 5 | Piezoelectric QCM, SAW, BAW or cantilever 
|
Change in resonant frequency | High sensitivity; fast response; good CMOS compatibility; scalability | Complex fabrication process; interference from humidity and temperature; low signal-to-noise ratio; unstable at higher temperature; complex readout circuitary | [40, 44, 45, 47] |
| 6 | Gas chromatography or similar techniques 
|
Analytes separated and detected using ionization method | High sensitivity; high selectivity | Bulky; expensive; time-consuming | [71–75] |
CMOS: complementary metal oxide semiconductor, QCM: quartz crystal microbalance, GC-MS: gas chromatography-mass spectroscopy, SAW: surface acoustic wave, BAW: bulk acoustic wave, FET: field effect transistor.
Other efficient techniques, such as gas chromatography (GC), gas chromatography-mass spectroscopy (GS-MS), proton transfer reaction-mass spectrometry (PTR-MS), selected ion flow tube technique-mass spectrometry (SIFT-MS), ion mobility spectrometry, and high-pressure liquid chromatography have been used to detect trace amounts of VOCs [71–75]. Most of these techniques have been successfully applied to the food industry, biomedical, and pharmaceutical areas. These instruments are capable of detecting trace amounts of chemicals in a batch, where chemical sampling, transportation, separation, detection, data transmission, and post-data analysis are time-consuming processes. However, due to limitations, such as bulkiness, high cost, and requirement of well-trained personnel to operate, these techniques may not be suitable for a low-cost portable E-nose system; thus, they are not discussed in detail.
2.2. Machine learning in E-nose technology
In an E-nose system, the output data from the sensor array is converted into an electrical signal pattern. Each sensor provides a dynamic response in terms of an electrical signal via weighting, standardizing, and normalizing, making it suitable for statistical analysis. The analyzed data is converted from higher to lower dimensions depending on the chemicals of interest. In general, data pattern analysis falls into three categories: graphical, multivariate, and neural network analysis [22]. Graphical analysis is one of the simplest forms of data analysis, where a sensor's response is analyzed using a bar plot or polar plot techniques. These graphical analyses are useful for visually interpreting a chemical signature with a specific reference. However, graphical analyses are not useful when high dimensional data is used. Multivariate data analysis is used to reduce the dimension of response data of complex chemicals by eliminating the possible disturbing variables, such as ambient temperature and humidity [76]. There are many multivariate analysis techniques, such as principal component analysis (PCA), linear discriminate analysis (LDA), discriminant function analysis (DFA), canonical discriminate analysis (CDA), and partial least squares regression (PLS regression), used in an E-nose system [23]. Amongst these techniques, PCA is routinely used in an E-nose for dimensionality reduction. PCA is an unsupervised learning technique, which is used to identify the chemicals without prior knowledge. However, the prime purpose of an E-nose is to identify chemicals qualitatively and quantitatively. Thus, it also requires classification techniques, such as support vector machines (SVM), k-nearest neighbor (k-NN), and neural network analysis [77]. These classification techniques are used in the E-nose system to identify chemicals based on mathematically trained models. If the results from neural network analysis and multivariate analysis show good agreement, then the confirmational accuracy of chemical identification gets better. Thus, ML has been employed in E-nose systems.
To date, various methods for sensor selection, data acquisition and pre-processing, dimensionality reduction, classifier choice, and validations have been used in E-nose systems. Li et al used different groups of sensor arrays (electrochemical, catalytic combustion, hot wire, and MOS sensors) and five different methods of ML to check the performance against the 95% confidence interval [78]. By considering 10-fold cross validation, they successfully concluded that LDA with fuzzy k-NN and LDA with SVM provided optimal classification performance. In general, Euclidean and Manhattan distances are considered for the k-NN method. Hariyanto et al carried out comparative classifier performance analysis of four different models: k-NN (k = 8) with the Canberra distance with and without normalization, SVM, and artificial neural network (ANN) [79]. In terms of model accuracy, k-NN with normalization provided an optimum result. E-nose system-based ML provided excellent differentiation between the disease and non-disease state. However, it did not demonstrate proper differentiation associated with the existence of different disease states with any confounding factors (age, sex, smoking status, and location of sampling site). Thus, Nakhleh et al used hierarchical clustering to check resemblance with such confounding factors and with a physiopathological group of diseases [80]. In 2014, Yan et al modified existing dimension reduction by sequential forward selection (SFS) and a classifier by SVM with model fusion (support vector regression). It was concluded that SFS provides a better result than current state-of-the-art PCA [81]. For classifier model development, the model fusion method trains the classifier with the local model (subject-specific) and global model (general available training data). It was observed that model fusion reduces the chances of over-fitting. Instead of LDA, Blatt et al used nonparametric linear discriminant analysis (NPLDA) and PCA for feature extraction. It was concluded that NPLDA feature extraction was better than PCA [82]. For pattern recognition studies, various classifier families, such as k-NN (classic, modified, and fuzzy), the discriminant functions classifier (LD: linear discriminant and QD: quadratic discriminant), and ANN, have been investigated. It was observed that if the response is not linearly separable, a quadratic discrimination function could be suitable. For binary classification, the gradient descent approach was implemented to obtain minimum cost function. In 2015, Leopold et al compared different dimension reduction, classification, and validation methods found in 46 publications on E-nose-based disease detection systems [83]. A few available E-nose technologies summarized by considering supervised sensitivity, selectivity, accuracy, p-value, and the mean absolute error are shown in table 3. It can be seen that no single combination of pattern recognition algorithms is superior to others. Various combinations of dimensionality reduction and classification techniques need to be applied to obtain optimum classification. These techniques have several advantages as well as disadvantages in terms of sensor technology, ML, and performance parameters. Additionally, none of these techniques have been fully adopted into clinical practice. With increasing numbers of research groups working on E-nose technology, it is envisaged that E-nose diagnostic tools have the potential to match, and even supersede, classical diagnostic methods. Although the E-nose has been studied for several diseases it has not been completely explored to its full potential. This collective information may be helpful in development of futuristic E-nose technology.
Table 3. A comparison between different E-nose systems with various dimension reduction, classification and validation processes for LC and diabetes detection.
| References | No. of Patients / Breath Samples | Diseases | Sensor Array Type | Dimensionality Reduction | Classifier | Validation | Results |
|---|---|---|---|---|---|---|---|
| [79] | 40 breath samples (20 healthy subjects + 20 diabetes patients) | Diabetes | Electrochemical gas sensors | Normalization, DWT | k-NN (k=8), ANN, SVM | Usability testing | 95.0% accuracy, |
| 91.3% sensitivity, 94.12% specificity, | |||||||
| 0.898 kappa value for k-NN | |||||||
| [81] | 36 (203 breath samples) | Diabetes | 9 MOS sensor + 1 CO2 sensor | SFS, PCA | SVR (Fusion Model) | Cross validation | Mean absolute error was lowest in case of SFS/SVR |
| [84] | 445 (108 healthy + 117 diabetes patients + 110 renal disease patients + 110 airway inflammation patients) | Diabetes and other malignancy | Chemical sensors | PCA | k-NN (k = 5) | Internal validation | 87.67% sensitivity & 86.87% specificity for diabetes, |
| 86.57% sensitivity & 83.47% specificity for renal disease, | |||||||
| 70.20% sensitivity & 75.07% specificity for airway inflammation | |||||||
| [80] | 1404 (813 suffering from any of 17 diseases + 591 healthy subjects) | 17 (cancerous, inflammatory, and neurological diseases) | Molecularly modified Au nanoparticles and a random network of single-walled carbon nanotubes | Raw sensor values | DFA, clustering | Cross validations 1. LOOCV 2. Blind validation (77% − 23% for training and validation, respectively) | Average accuracy among 120 DFA is 86% |
| [78] | 52 (24 LC patients + 5 other respiratory disease patients + 10 healthy smokers + 13 healthy non-smokers) | LC | 9 MOS + 3 electrochemical sensors + 1 catalytic combustion + 1 hot wire gas sensor | PCA, LDA, LE, LLE, tSNE | fuzzy k-NN, SVM | 10-fold cross validation | 91.59% accuracy, |
| 91.6% sensitivity, | |||||||
| 91.72% specificity, | |||||||
| 95% CI | |||||||
| [82] | 101 (58 healthy + 43 LC patients at different stages) | LC | MOS | NPLDA, PCA | Variants of k-NN, LD, QD, BPANN | Cross validation | 92.6% accuracy, |
| 95.3% sensitivity, | |||||||
| 90.5% specificity | |||||||
| [85] | 335 (79 healthy + 165 LC + 91 other disease patients) | LC | Cyranose 320 | Raw sensor values | SVM | Cross validation | 77.3% for cancer versus non-cancer model, |
| 91.6% for cancer versus healthy volunteer's model | |||||||
| [86] | Not reported | LC ED | GC-MS + Cyranose360 + colorimetry + NanoNose (chemresistor with gold nanoparticles) | PCA | Fisher's LDA | Not reported | 87% accuracy for GC-MS, |
| 85% accuracy for Cyranose360, | |||||||
| 73.3% sensitivity and 72.4% specificity for colorimetry, | |||||||
| >90% reproducibility for NanoNose | |||||||
| [87] | 30 (20 LC patients, 10 other lung disease patients) | LC | QCM | Raw sensor values | PLS-DA | Internal validation | >90% accuracy |
| [88] | 89 (33 non-smokers, 11 ex-smokers, 18 smokers, 11 patients with respiratory disorders, 16 LC patients) | LC | Chemical (ENS Mk3 module) | PCA, analysis of variance | SPSS 12.0 software package | Not reported | p-values observed for different channels are 0.025, 0.045, and 0.001 |
| [89] | 32 (10 healthy + 15 LC patients + 7 CB patients) | LC ED | SAW | Raw sensor values | BPANN | Validated with 8 subjects | Accuracy not reported |
| [90] | 50 (18 healthy + 24 LC patients + 8 CB patients) | LC | SAW | Raw sensor values | BPANN | Not Reported | Sensitivity of decane is 2.12 ng Hz−1, |
| accuracy not reported | |||||||
| [91] | 146 (76 healthy + 70 LC patients) | LC | Quartz microbalances | Raw sensor values | PLS-DA | LOOCV | 81% sensitivity, |
| 91% specificity for stage-I | |||||||
| [92] | 475 (223 healthy + 252 LC patients) | LC | Cyranose 320 | Raw sensor values | LRA | Not reported | 95.8% sensitivity & 92.3% specificity for smokers, |
| 96.2% sensitivity & 90.6% specificity for non-smokers |
MOS: metal oxide semiconductor, SFS: sequential forward selection, PCA: principal component analysis, SVR: support vector regression, DWT: discrete wavelet transform, k-NN: k-nearest neighbor, ANN: artificial neural network, SVM: support vector machine, DFA: discriminant factor analysis, LOOCV: leave-one-out cross validation, CB: chronic bronchitis, LC ED: lung cancer early detection, BPANN: back propagation artificial neural network, , PLS-DA: partial least squares discriminant analysis, LDA: linear discriminant analysis, LE: Laplacian Eigenmap, LLE: local linear embedding, tSNE: t-stochastic neighbor embedding, CI: confidence interval, NPLDA: nonparametric linear discriminant analysis, LD: linear discriminant, QD: quadratic discriminant, QCM: quartz crystal microbalance, GC-MS: gas chromatography-mass spectroscopy, SAW: surface acoustic wave, LRA: logistic regression analysis.
3. VOCs in disease conditions
To date, more than 3000 VOCs have been detected qualitatively and quantitatively in the exhaled breath. VOCs coming out through lung, kidney, sweat, or skin provide vital information regarding metabolic alterations or malfunctions occurring inside our bodies. Endogenous VOCs, which originate via internal metabolic processes, are beneficial from a clinical perspective as they carry information about the internal metabolic changes that may have occurred due to certain conditions. Exogenous VOCs, which originate from current or previous environmental exposure, may create hurdles during testing, but they hold great significance, as they may carry useful historic information about the patient's habits, such as smoking and environmental exposure. However, these have been sparsely studied to date. Finding the exact concentration of exhaled VOCs for medical interpretation is quite challenging. A typical concentration of endogenous VOCs varies from 1 ppb to 5000 ppb, as quantified by some advanced techniques [3]. These concentrations strongly depend on lifestyle, diet, age, gender, weight, smoking habits, the existence of different diseases, and medication use [93–98]. Most of the endogenous VOCs are divided into the following categories: saturated hydrocarbons, unsaturated hydrocarbons, oxygen-containing species, nitrogen-containing compounds, and sulphur-containing compounds. Several processes, such as lipid peroxidation, protein oxidation, or DNA damage caused by the chemical interactions of inflammatory cells and reactive oxygen species, produce hydrocarbons [99]. Other groups of VOCs in the breath, such as alcohols, aldehydes, acids, esters, and ketones are evolved via different biochemical and physiological processes. Abnormal levels of VOC formation indicate a different metabolic status that can be used to differentiate between healthy and diseased individuals. Also, a patient's treatment progress can be visualized by monitoring these VOCs using technologies such as the E-nose. One major challenge for implementation of E-nose technology is breath sampling. Contamination by background VOCs or improper sampling methodology will result in false positive results [100]. To obviate these issues, recommended guidelines, such as the European Respiratory Society (ERS) technical standard document on breath sampling, are followed for best results [101]. For breath sampling several factors, such as inhalation of filtered or unfiltered air, single or multiple exhalation, and whether the collected breath is mixed expiratory or end-tidal, are considered [102–106]. The expiratory flow rate, breath-hold time, and anatomic dead space also influence the pattern of VOCs, as studied by Bikov et al and Boshier et al [107–109].
4. Breath analysis for diabetes
4.1. Background
Diabetes is an insulin disorder in which a patient has a high blood glucose level. A diabetic patient's pancreas either does not produce enough insulin or does not use insulin effectively. According to pathological mechanisms, diabetes is classified into three categories, namely type-I, type-II and gestational diabetes mellitus (GDM) [110]. Type-I diabetes is due to less production of insulin in the body, i.e. the majority of insulin-producing beta-cells are destroyed due to an autoimmune reaction, thus patients require externally injected insulin. In type-II diabetes, insulin is not properly used by the glucose-consuming cells. GDM is an extension of diabetes from mother to child, where both mother and child are susceptible to suffering from type-II diabetes in the future. Along with these three major types, other diabetes, such as monogenic diabetes like neonatal diabetes and maturity-onset diabetes of the young (MODY), and drug or chemical induced diabetes are found in the human body [111–113]. The sweet smell of the diabetic patient's breath was observed a long time ago, but the exact reasons were not known. Later, people observed that diabetic patients have elevated ketone body (acetone, hydroxybutyrate, and acetoacetate) concentrations. These ketone bodies are the byproducts of metabolized fatty acids. In diabetic patients, fatty acids are metabolized (alternative energy source) instead of normal glucose metabolism to produce ketones. The acetoacetate is derived from lipid peroxidation, which generates the body's energy source beta-hydroxybutyrate [114]. The formation of acetone in exhaled breath is due to non-enzymatic decarboxylation of acetoacetate. The details of acetone formation from fatty acids are schematically shown in figure 3. The emitted acetone is absorbed with the bloodstream and expired via the lungs with the breath along with other normal constituents of breath. However, ketone formation is quite complex as they are produced during ketogenic diets as well as in the fasting state, i.e. during insufficient glucose availability.
Figure 3. A schematic representation of acetone formation from fatty acids. Adapted from [114]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageThe elevated breath acetone concentration in a diabetic patient linearly depends on the blood glucose concentration, as reported by Wang et al [115]. However, some authors have contradicted the clear relationship between breath acetone and the blood glucose level. This is because acetone generation strongly depends on several factors, including age, gender, sampling before/after meals, nature of the meal, amount of meal, whether the subject is active in sports, whether a habitual smoker or not, alveolar versus non-alveolar air during sample collection, and presence of other disease conditions in patients [114, 116, 117]. The typical breath acetone concentration in the diabetic patient is over 1.7 ppm, which is at least two times higher than a normal healthy person [118]. A breath VOC-based screening method obviates several shortcomings of urine VOC and blood-based methods. Urine-based methods are less sensitive to beta-hydroxybutyrate (which appears early), will give the average number of ketone concentration from the last void if not catheterized, and are not reliable if the patient has renal failure, is suffering from renal conditions, or has difficulty passing urine [119]. Blood-based methods are invasive, require trained staff and optimum sterilization, and the VOC concentration is affected by other components, such as vitamin C dissolved in the blood [120, 121].
4.2. E-nose technology for diabetes
Quantification of breath acetone is quite challenging due to interfering water vapour and VOCs such as ethanol, ethylbenzene, methyl nitrate, and xylene. In most cases, expensive and complicated techniques, such as GC-MS, SIFT-MS, PTR-MS, and cavity ringdown spectroscopy, are used to measure breath acetone concentrations. From a commercial point of view, the breath analyzer should be portable, enable real-time monitoring, accurate, smaller in size, and low-cost. In this regard, few successes have been achieved in the development of highly sensitive individual gas sensors and E-nose systems using an array of gas sensors to detect diabetes. In 2006, Lu et al used an array of 32 sensors with single-walled CNTs decorated by different metal nanoparticles and polymers for acetone detection. The sensor response was processed using PCA to discriminate acetone from NO2, HCN, HCl, and Cl2 with potential applications in diabetes detection [122]. In 2002, Mohamed et al developed an E-nose tool for diabetes screening using an array of eight sensors and PCA [123]. This tool successfully (96%) classified type-II diabetes by considering the smell of the patient's urine sample. Ping et al developed an E-nose device consisting of different metal catalyzed SnO2 sensor arrays [124]. The device was able to diagnose diabetes correctly by testing breath samples of 32 volunteers (18 diabetic and 14 healthy subjects), before and after taking meals. In 2012, Yan et al used nine commercial sensors, including a temperature and humidity sensor, to discriminate diabetes with 91.43% sensitivity and 89.86% specificity [125]. Instead of the breath sample, Siyang et al studied the urine sample to identify the glucose concentration in diabetes patients using an E-nose embedded with eight Taguchi gas (TGS) sensors [126]. Guo et al used 12 commercial TGS sensors and an ML system to diagnose diabetes, renal failure, and airway inflammation disease [84]. The diabetes subjects were correctly diagnosed with 87.67% sensitivity and 86.87% specificity. Further, they used the same 12 sensors to monitor blood glucose levels of diabetic patients with 68.66% accuracy [127]. Although this prediction is not prominent, it was probably the first portable E-nose tool developed for blood glucose monitoring. In this tool, the support vector ordinal regression (SVOR) algorithm was used for blood glucose monitoring. Instead of a TGS sensor and SVOR algorithm, Saraoğlu et al used a QCM sensor and a radial basis function neural network for blood glucose and HbA1c monitoring [128]. Nine different phthalocyanine coated QCM sensors were used for a glucose and HbA1c parameter study. Their system showed improved accuracy (74.76%) during the blood glucose monitoring study. By considering a subject-specific prediction model in PCA, Yan et al developed a highly accurate (>90%) diabetes screening and blood glucose monitoring E-nose system [129]. A portable E-nose system consisting of an array of conductive polymer (polypyrrole) sensors having different thicknesses was developed by Yu et al to discriminate diabetic patients from healthy persons [130]. Recently, Leopold et al carried out glucose monitoring of critically ill patients admitted to ICU using E-nose technology [131]. Four metal oxide gas sensors and PCA along with a linear regression model was used in this E-nose system to monitor the blood glucose level. Leopold et al concluded that the E-nose itself cannot predict exact blood glucose levels in specific cases of intubated ventilated ICU patients.
4.3. Nano-sensors for diabetes
To reduce complex signal processing or pattern recognition in the E-nose tool, researchers have focused on individual sensors, specifically its sensing materials, to detect the sub-ppm concentration of acetone. Over the last few decades, individual semiconducting metal oxide (SMO) (SnO2, ZnO, WO3) and its engineered structures have been used to detect acetone. For example, WO3 nanofibers with a porous morphology show excellent response and selectivity towards acetone vapours [132]. However, selective sub-ppm acetone detection is quite challenging at a low operating temperature and thus, most researchers have focused on composite, doped, or functionalized materials with MOS (especially WO3, as it detects acetone selectively) for detecting sub-ppm concentrations of acetone. For example, Tomer et al and Choi et al used In/WO3-SnO2 nanocomposites and Pt-functionalized WO3 hemitube material to detect sub-ppm concentrations of acetone [133, 134]. Wang et al have shown that 10 atom % Cr-doped ε-WO3 nanoparticles are highly sensitive and selective towards low concentrations of acetone [135, 136]. It is known that the exhaled breath contains ∼90% relative humidity (RH), which hampers exhaled breath measurements. In 2016, Kim et al used electrospun WO3 nanofiber functionalized by Rh2O3 nanoparticles to detect 1 ppm of acetone in a 95% RH environment [137]. Similarly, carbon doped WO3 synthesized by a template method has shown excellent acetone sensing characteristics in terms of sensitivity, selectivity, and response time in the RH environment [138, 139]. However, significant success in sub-ppm acetone sensing was achieved by Righettoni et al using Si-doped WO3 prepared by spray pyrolysis. They varied the Si-doping content to achieve optimized acetone sensing results (at 10 mol% Si-doped WO3) in dry as well as in the RH environment, as shown in figure 4(a) [140, 141]. The results show the sensors ability to delineate diabetes subjects from healthy subjects (figure 4(b)). The same type of sensor was used for real-time breath acetone concentration measurement, which was further verified using a PTR-MS detector and showed 98% accuracy [142]. Additionally, their sensor showed a reasonable response when tested against different flow rates, which is equivalent to different patients' breathing conditions. One of the biggest disadvantages of spray pyrolysis is its non-uniformity over a larger scale and thus, Rydosz et al used a relatively simple technique (sputtering) to deposit 1 at% Si-doped WO3 for detection of sub-ppm concentrations of acetone [143]. However, all these Si-doped WO3 sensors showed an optimal response at a relatively high temperature (∼400 °C).
Figure 4. (a) Acetone response characteristics of a WO3-based sensor with respect to the Si-doping content and different RH levels toward 600 ppb acetone at 400 °C. (b) Acetone response characteristics of 10 mol% Si-doped WO3 nanoparticles in a concentration range of 0–3 ppm with 90 % RH at 400 °C. Reprinted with permission from [141]. Copyright (2010) American Chemical Society.
Download figure:
Standard image High-resolution imageFor the purpose of diabetes diagnosis, Shin et al and Narjinary et al used Pt-decorated porous SnO2 fibres and SnO2-CNT nanocomposites to detect acetone vapour. These materials showed excellent sensitivity (few ppb levels of acetone in a high RH environment) [144, 145]. Recently, graphene/graphene oxide/reduced graphene oxide-SMOs have attracted a great deal of interest for acetone detection with potential diabetes diagnostic applications. Choi et al used SnO2 nanofibers functionalized with reduced graphene oxide nanosheets and Co3O4 nanofibers with Ir nanoparticles and graphene oxide for acetone detection with a potential diagnosis of diabetes [146, 147]. They also developed Pt-functionalized WO3 hemitubes for diabetes diagnosis by measuring trace amounts of acetone vapours in an 85% RH environment [134]. Although success has been achieved in certain areas, such as sensitivity, selectivity, response time, and low power consumption (low operating temperature), significant work needs to be carried out for large-scale production of sensors and its automatization for diabetes screening. Additionally, there is a need to develop an ML algorithm for accurate prediction of breath acetone intensity and blood glucose level. In-depth study of these breath analyzing tools will be helpful in clinical studies for large-scale diabetes screening and its therapy control in a quick and non-invasive way.
5. Breath analysis for lung cancer
5.1. Background
Lung cancer is listed amongst the most common cancer and is one of the leading causes of cancer death [11]. The survival rate of stage-I lung cancer is far better than later stages, and thus an early diagnosis of lung cancer is desirable for better survival and prognosis [148]. Common methods for lung cancer detection are CT and bronchoscopy. Also, sputum cytology, biopsies, and polymerase chain reaction (PCR) sputum assays have been used to detect lung cancer [149–151]. The sensitivity of a CT scan is weak, whereas bronchoscopy is a minimally invasive technique and many times requires the administration of short-term anesthesia, which at times is associated with complications and trauma to patients [152, 153]. Imaging techniques such as MRI and CT scans are indeed well established and accepted as a proven gold standard [152, 154]. From a mass screening perspective, they have their inherent limitations, such as high equipment cost, considerable expenditure on maintenance, dedicated infrastructure, requirement of trained personnel, and for the patient to visit a dedicated facility. Comparatively, the E-nose is a non-invasive point-of-care platform that can be easily operated by a semi-skilled person, and thus will potentially aid in large-scale screening scenarios. Thus, much research is focused on developing a simple diagnostic method, such as the E-nose, where lung cancer can be detected in a non-invasive way in less time. In 1985, Gordon et al put forth the list of VOCs associated with lung cancer [155]. Using GC techniques, several VOCs such as benzene, toluene, styrene, decane, isobutane, methanol, ethanol, acetone, pentane, isoprene, isopropanol dimethylsulfide, 2-methyldodecane, 2-tridecanone, 2-pentadecanone, eicosane, SCC-2-decanone, 2-hendecanone, 2-methylnaphthalene, nonadecane, eicosane carbon disulfide, aldehyde-butanal, formaldehyde, acetaldehyde, pentanal, hexanal, and octanal to name but a few, are found to be present in a lung cancer patient's breath [156–161]. The concentration of these VOCs is found to be elevated in a lung cancer patient's breath compared to a healthy subject's breath [162]. These elevated VOCs or the production of new VOCs in patients are due to metabolic disorders. A normal or healthy human body tends to maintain the plethora of VOCs discussed, in definite ratios. Metabolic disorders and other pathological conditions can either change this ratio or even produce new VOCs, which also in turn changes this ratio. Cells in their normal state produce reactive oxygen species (ROS) at a definite rate, owing to the oxidative stress that results from routine cell activities [163]. The ROS leaks from the mitochondria or the fatty acids in the cell membrane to the cytoplasm. The leaked ROS then acts on the organic material in the cytoplasm and produces different volatile alkanes, which are exhaled through the breath as VOCs, in normal conditions [164]. During cancer, the cells are proliferating and survive in a condition of hypoxia through impaired mitochondrial respiration and a higher rate of glycolysis (Warburg effect) leading to a generally acidic microenvironment [165]. This environment helps break the basement membrane, while keeping the immune cells at bay and increases the metastatic potential of the malignant transformation at the site in question [166]. Simultaneously, genetic mutations also occur: for example, the increased expression of mixed oxidase enzymes, such as cytochrome p450, known to be associated with tobacco smoking and lung cancer. This over activation of p450 through genetic and microenvironment changes during malignant transformation, accelerates the catabolism of the oxidative stress products that normally creates VOCs, thereby leading to their increased abundance and hence modified ratios in the exhaled breath [167]. This can thus be picked up as a potential signature for lung cancer, as schematically represented in figure 5.
Figure 5. A schematic representation of a hypothetical pathophysiological model as a basis for the breath test based on VOCs for lung cancer detection. This hypothesis is adopted from [161].
Download figure:
Standard image High-resolution imageBesides the GC-MS technique, relatively inexpensive and reliable techniques, such as ion mobility spectrometers and Raman scattering, have been used to detect lung cancer related VOCs [168, 169]. In 2006, McCulloch et al used five trained dogs to sniff breath samples of 55 lung cancer subjects with 99% sensitivity and 99% specificity [170].
5.2. E-nose technologies for lung cancer
With demand for an inexpensive, portable, and quick measurement system, researchers have focused on E-noses such as the commercially made Cyranose 320 and sensor array embedding colorimetric sensor, QCMs, SAWs, CNTs, and gold nanoparticles. In 2005, Machado et al detected lung cancer in 14 out of 76 subjects using the Cyranose 320 E-nose with 71.4% sensitivity and 91.9% specificity [156]. The electronic nose had 66.6% positive and 93.4% negative prediction. Similarly, Dragonieri et al used the Cyranose 320 to discriminate lung cancer patients from chronic obstructive pulmonary disease (COPD) patients and normal subjects [171]. Smoking is a critical disturbing factor for lung cancer detection using an E-nose system, and thus, McWilliams et al used the same Cyranose 320-based two E-nose system to differentiate between a lung cancer patient's breath and a high-risk smoker's breath with 80% accuracy [172]. Like Cyranose 320, Tran et al used the ENS Mk-3 E-nose for discriminating lung cancer breath from a normal subject's breath [88].
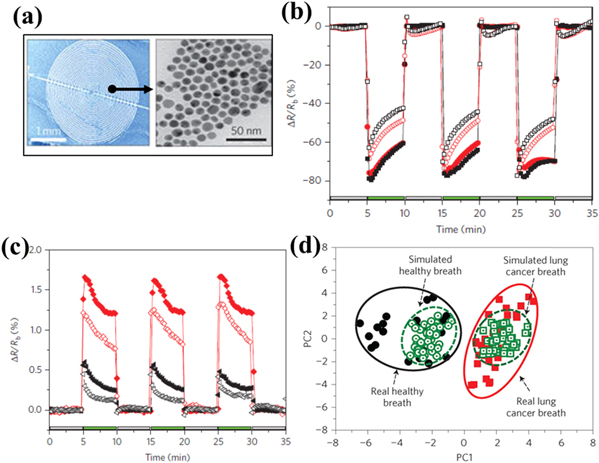
Another technique known as the colorimetric sensor array is a disposable cartridge with 36 dots, which are filled with chemically sensitive compounds. Absorption of VOCs leads to a change in the colour of each dot. Mazzone et al demonstrated lung cancer detection using a colorimetric sensor array with 73.3% sensitivity and 72.4% specificity from several control subjects, including idiopathic pulmonary fibrosis, pulmonary arterial hypertension, COPD, sarcoidosis, and healthy subjects [173]. They further improved the accuracy of the model by considering smoking status, age, and the sex of each subject [174]. Similarly, Huo et al used a colorimetric sensor array with Au nanorod-MTPP polymers as sensing materials to detect lung cancer [175]. For lung cancer screening, they successfully detected six VOCs: decane, undecane, hexanal, heptanal, benzene, and 1,2,4-trimethylbenzene. Di Natale et al used eight QCM sensor arrays coated with different metalloporphyrins to detect lung cancer with 100% sensitivity and 94% specificity [176]. This method showed a successful delineation of 42 lung cancer subjects from 69 subjects with 90.3% accuracy. In 2010, D'Amico et al used the same E-nose sensor array to discriminate between lung cancer and other lung-related diseases like COPD, bronchitis, pleurisy, and interstitial lung with 93% sensitivity and 79% specificity [177]. A quartz microbalance sensor's sensitivity is relatively low and also the availability of metalloporphyrin thin film with different selectivity is unmanageable for the large-scale sensor array. Thus, researchers have focused on the alternative piezoelectric-based gas sensor for better precision. In 2005, Chen et al studied an E-nose based on SAW sensors to discriminate lung cancer from normal subjects by considering 11 VOCs as biomarkers [90]. Similarly, Wang et al considered five VOCs as the best combined biomarkers of lung cancer to discriminate from normal subjects [178]. The same group also used a SAW-MOS sensor array on 89 clinical samples to detect lung cancer with 93.62% sensitivity and 83.37% selectivity [179]. With demand for small-sized, easily operable, and portable E-noses, researchers have focused on chemiresistive-type sensors to detect VOCs. Peng et al developed an array of sensors with single-walled CNTs coated with ten different nonpolymeric organic materials as sensing materials to detect lung cancer [180]. Their E-nose successfully discriminated between lung cancer patients' VOCs and healthy subjects' VOCs at low RH values. Their results were further confirmed by GC-MS. Similarly, Liu et al used single-walled CNTs coated with tricosane (C23H48) and pentadecane (C15H32) materials to detect lung cancer biomarkers, such as 1,2,4-trimethylbenzene and decane [181]. Also, Castro et al and Chatterjee et al used CNTs and five different conductive polymer nanocomposites to detect lung cancer biomarkers [182, 183]. Prof. Haick's group have published articles on gold nanoparticle-based VOC sensors to detect lung cancer [184–187]. The group used nine different chemiresistive sensors embedded with 5 nm size gold nanoparticles with different organic functionalities (dodecanethiol, decanethiol, 1-butanethiol, 2-ethylhexanethiol, hexanethiol, tert-dodecanethiol, 4-methoxytoluenethiol, 2-mercaptobenzoxazole and 11-mercapto-1-undecanol). The change in resistance of each sensor was measured after exposure to exhaled breath for 5 min and further processed by PCA, as shown in figures 6(b)–(d) [184]. Their sensor array could distinguish the breath of stage III and stage IV lung cancer patients from the breath of healthy subjects after preconcentration or dehumidification of breath samples. Further, they developed a similar type of 18 nanosensor array to detect non-small-cell lung cancer (early stage cancer) with 100% sensitivity and 75% specificity, despite not carrying out preconcentration of breath samples [185]. In 2010, they considered a 14 nanosensor array to detect different cancers. Their study showed a clear distinction between patients with lung, colon, prostate, and breast cancers from healthy subjects [186]. The same group worked on an E-nose with an 18 sensor array (16 sensors were based on gold nanoparticles with different organic functionalities and two sensors were based on CNTs with polycyclic aromatic hydrocarbons) to detect malignant and benign disease. They were able to discriminate between adenocarcinomas and the squamous cell carcinomas with 88% accuracy [187]. By combining GC-MS and a nanosensor array, Agmon et al developed an E-nose system to supervise anticancer treatment of advanced lung cancer [188].
Figure 6. (a) An image of a gold nanoparticle-based resistive sensor. (b) The response towards healthy breath when 11-mercapto-1-undecanol-gold nanoparticles (red filled circles) and decanethiol-gold nanoparticles (black filled squares) were used as sensing elements, and the response towards a lung cancer patient's breath when the responses of 11-mercapto-1-undecanol-gold nanoparticles (red open circles) and decanethiol-gold nanoparticles (black open squares) are used as the sensing element. The inset image shows an image of the device along with a transmission electron microscopy (TEM) image of gold nanoparticles. (c) The response towards healthy breath when 2-mercaptobenzoxazole-gold nanoparticles (red filled diamonds) and tert-dodecanethiol-gold nanoparticles (black filled triangles) are used as sensing materials, and the response towards a lung cancer patient's breath when 2-mercaptobenzoxazole-gold nanoparticles (red open diamonds) and tert-dodecanethiol-gold nanoparticles (black open triangles) are used as sensing materials. (d) PCA of the response dataset towards real and simulated breath to discriminate between a healthy person's breath and a lung cancer patient's breath. Reprinted by permission from Macmillan Publishers Ltd: Nature Nanotechnology [184], Copyright (2009).
Download figure:
Standard image High-resolution image5.3. Nano-sensors for lung cancer
Along with the E-nose system, researchers have focused on individual sensors to detect particular VOCs related to lung cancer. For example, a very low concentration of toluene was detected using thin-walled SnO2 nanofibers functionalized with Pt nanoparticles and porous WO3 nanofiber with a Pd catalyst [189, 190]. These low concentration toluene detectors demonstrate possible application in lung cancer diagnosis. Wu et al demonstrated a unique disposal lung cancer diagnosis tool embedded with four sensors (two TiO2 and two Ag NWs) [191]. These sensors are used to measure the change in humidity, temperature, flow rate, and the lung cancer biomarker 2-propyl-1-pentanol.
6. Summary and future outlook
Since the discovery of VOCs in exhaled breath, intensive research has been carried out to detect biomarker VOCs. It has been found that different metabolic activities in our body produce several VOCs and they are strongly correlated with specific disease states. To detect these VOCs in the breath several techniques, such as GC-MS, SIFT-MS, PTR-MS, optical-type, piezoelectric-type, and chemiresistive-type gas sensors, are used. Since the development of these techniques, many improvements have been made from a methodological point of view or sensing material point of view to produce a fast, low-cost, and portable device. However, current knowledge is enough to mimic but not enough to replace the mammalian nose. The available systems are application-specific tools and are not universal due to either a lack of sensitivity, selectivity, portability, or speed. Thus, a compact and portable E-nose system with high sensitivity, accuracy, precision, and fast and low detection limits is desirable. In this regard, few feats have been achieved in the development of E-nose systems by combining nonspecific sensor arrays and ML for different applications. Breath fingerprint analysis using the E-nose has great potential for diagnosing several diseases. Wang et al reported that acetone is a key biomarker found in diabetes patients' exhaled breath [114]. Promising results for the diagnosis of diabetes were achieved by using the E-nose system. Also, few researchers have used individual sensors to detect sub-ppm levels of acetone to diagnose diabetes. On the other hand, lung cancer patients' breath was successfully discriminated using an E-nose system embedded with different chemiresistive sensors. Unfortunately, most of these devices are still at the prototype stages and far from being translated to a commercial product due to limits in miniaturization, calibration, and large-scale production.
The demand for the E-nose for clinical studies, as well as for other studies, has been growing over the years. So far, there have been only a handful of studies in the E-nose system for diabetes and lung cancer. With an advancement in sensing devices, electronics, and signal processing, the E-nose system's size can be minimized along with fast data processing and can provide real-time results. These tools can fit into advanced systems, such as a human robot, or can fit into mobile technology for personal use, such as daily monitoring of the progress of a disease state or early detection of a disease state. E-nose systems have the capabilities to detect diseases at early stages, which will be helpful to patients psychologically and economically. In a matter of time, we will see the E-nose as a non-invasive technology for early diagnosis of many diseases.
Acknowledgments
Bhagaban Behera acknowledges the Science and Engineering Research Board (SERB) of the Government of India for providing financial assistance (National Postdoctoral Fellowship, File number: PDF/2017/000644). Hardik J Pandya acknowledges the Indian Institute of Science, Bangalore for the start-up grant to establish the research and computational facilities at the Department of Electronic Systems Engineering. The authors also extend their gratitude to Mansi Saxena, Arun Baby, and Darshan Parsana for providing valuable feedback during the article preparation.
Conflict of interest
The authors declare that they have no conflict of interest.