Written by Magnus Nossen
The patient in today's case is a male in his 70s with hypertension and type II diabetes mellitus. His wife contacted the ambulance service after the patient experienced an episode of loss of consciousness. The syncope lasted about 2-3 minutes according to his wife. He woke up alert and with chest pain which he also had experienced intermittently over the previous few days. The ECG below was recorded about 20 minutes after he regained consciousness. What do you think?
ECG #1
The above ECG shows sinus rhythm at about 60 bpm. There is RBBB and LAHB. The PR interval is normal. The first task when assessing a wide complex QRS for ischemia is to identify the end of the QRS. When you have identified the end of the QRS complex and the beginning of the ST segment you can assess whether there is concordant ST segment elevation, concordant ST depression or whether there are excessively discordant ST segments. Below (Figure A) I have marked the end of the QRS complexes by drawing a vertical line through the J-point of the standard and precordial leads. Blue arrows indicate ST depression and the red arrow ST elevation.
Figure A
It now becomes apparent that there is ST segment depression in almost every lead of the ECG (V1-V6, I, II, aVL and aVF). The ST segment in lead III is close to isoelectric, with perhaps slight ST elevation. There is ST elevation in lead aVR as expected with these widespread ST depressions. The ST segment depression in the precordial leads is excessively discordant. In leads I, II and aVF there is concordant STD.
This ECG has widespread ST depression and an almost "Aslanger-like" appearance. The ST segment changes are compatible with severe subendocardial ischemia which can be caused by type I MI from ACS or potentially from type II MI (non-obstructive coronary artery disease with supply/demand mismatch).
There are multiple possible clinical situations that could account for diffuse subendocardial ischemia that is not due to ACS and plaque rupture. The history in today's case with sudden loss of consciousness followed by chest pain is very suggestive of ACS and type I ischemia as the cause of the ECG changes. In my experience, patients having a type II MI (unless caused by a tachydysrhythmia) usually have a more gradual onset of symptoms reflecting the more gradual onset of most common clinical entities associated with type II MI (e.g sepsis, anemia, hypoxemia, severe hypotension etc., etc.)
The patient was given aspirin and heparin. While preparing for transport the patient became ashen and confused with cool and clammy skin and a very weak pulse. A repeat ECG was recorded about 15 minutes after the initial ECG. What do you think has happened and what is the most likely diagnosis?
Smith: after publication, Pierre Taboulet, who has an amazing French language ECG site, notified that he sees high grade AV block in this ECG:
ECG #2
Again there is a wide complex QRS due to RBBB and LAFB. The rhythm now is atrial fibrillation. In the initial ECG (ECG# 1) aVR had ST elevation. Now this lead shows STD depression. Many of the leads that showed ST depression in the initial ECG now show ST elevation! There now is marked ST elevation in leads I, aVL and V2-V6.
In Figure B below I have again marked the J point by vertical lines. The red arrows point to ST segment elevation.
Figure B
At this point, with the ECG changing from diffuse ST depression to widespread ST elevation and the patient presenting in cardiogenic shock, left main coronary artery (LMCA) occlusion is the likely diagnosis.
There is «shark-fin-like» ST elevation in many leads. This is an ominous sign. The patient was rushed to the nearest emergency department (non-PCI facility) for stabilization. Just prior to arrival he fell out of consciousness with the below ECG on the monitor.
ECG #3
ECG #4
Although the above ECG shows widespread and massive ST depression, these ischemic changes actually represent an improvement over the pre-thrombolytic ECG. (ECG #2). There is now some flow in the LMCA!
This series of ECGs (ECG #1, #2 and #4) illustrate well that subtotal occlusion of the left main is usually associated with severe subendocardial ischemia (profound and diffuse ST depression), whereas an ECG recorded during total occlusion of the LMCA (ECG #2) will show widespread ST elevations. Complete LMCA occlusion is associated with clinical shock and/or cardiac arrest.
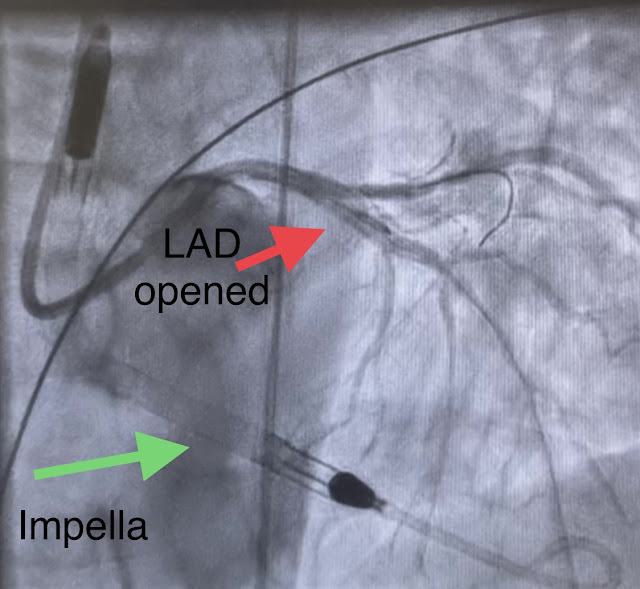
As mentioned above, some flow was restored in the left main coronary artery at the time of recording of ECG #4. Transport to a PCI-capable facility was arranged - and on arrival at the PCI centre, the patient maintained a mean arterial pressure (MAP) of 40-50 mmHg and was alert. His lactate was down to 8. The video below shows the coronary angiography. The image quality is not the best, but you can see the subtotal occlusion of the left main artery.
Post PCI an intra-aortic balloon pump (IABP) was placed and a combination of norepinephrine and dobutamine was needed to maintain perfusion pressures. Pressors could gradually be tapered within 24 hours. The initial troponin I drawn in the local emergency department prior to transfer to PCI center was 42ng/L (ref < 34ng/L) NT-proBNP was 307ng/L (ref < 300ng/L)
The below ECG was recorded 12 hours after PCI. There is sinus rhythm with a premature atrial contraction (P wave best seen in leads V1 and V2 superimposed on the T of the second QRS) The PAC conducts to the ventricle with a prolonged PR interval. The profound ST segment and T waves changes are gone. The bifasicular block persists
ECG #5
(12 hours after PCI)

High sensitivity troponin T 16 hours after admission measured 14.877ng/L (this is a massive infarct!). NT-proBNP was 3753ng/L There was transient liver enzyme elevation as is common with acute shock. The patient spent a couple of days in the cardiac intensive care unit receiving treatment for acute heart failure and aspiration pneumonia. He was later transferred back to his local hospital neurologically intact and without serious sequela. Long term follow up is not available.
How did the Queen of Hearts do on today's ECGs?
See the the below image with interpretation of ECG #1, #2, #4 and #5
See this post for 8 cases of total LM Occlusion:
How does Acute Total Left Main Coronary occlusion present on the ECG?
See this case and this case for more examples of ACS involving the LMCA
Learning points:
- LMCA occlusion carries a poor prognosis, most patients do not make it to the hospital. Those who make it to the ED usually have transient occlusions with reperfusion.
- Suspect LMCA with sudden shock and widespread ST elevations.
- Some patients are too unstable for transfer. Thrombolytics can be life saving, the patient in today's case likely would not have survived had he not been given thrombolytic therapy
- As per Dr. Nossen — it will not be often that emergency providers encounter patients with acute LMain occlusion — simply because survival of most of these patients is so limited. Today's case offers a unique opportunity to track the evolution of a patient during the process of ongoing LMain occlusion.
- There is no “single” ECG presentation for patients with acute LMain occlusion. Quite literally — You can see almost anything!
- The reason for this highly variable ECG presentation, is that multiple territories may be involved to varying degrees — making it impossible to predict how much ST elevation you will see — and how much opposing (reciprocal) ST depression will attenuate (if not completely cancel out) these initial ST segment vector forces.
- The ST-T wave appearance in lead aVR can be anything when there is acute LMain occlusion.
 |
| Figure-1: Reasons for the varied ECG presentation of acute LMain occlusion — excerpted from Dr. Smith’s 8/9/2019 post (This Table from My Comment in the January 16, 2020 post). |
- As per Dr. Nossen — today's initial ECG (LEFT tracing in Figure-2) shows sinus bradycardia with QRS widening due to bifascicular block (RBBB/LAHB).
- There is marked, diffuse ST segment depression in ECG #1. To facilitate distinguishing between the end of the QRS and the beginning of the ST segment — I highlight the J-point with BLUE arrows.
- Marked ST elevation is seen in lead aVR (to the right of the RED arrow in this lead).
- As we have noted on many occasions — this ECG picture of diffuse ST depression with ST elevation in lead aVR suggests DSI (Diffuse Subendocardial Ischemia). That said, more than just DSI — ECG #1 also demonstrates the bifascicular block (of RBBB/LAHB) that is so commonly seen in association with acute LAD occlusion.
- Another interesting feature seen in ECG #1 — is that lead III shows a nearly isoelectric baseline. I suspect this is the result of the interplay between electrical forces favoring ST elevation and ST depression.
- To Emphasize: DSI does not indicate acute infarction. And although severe underlying coronary disease is often the cause — non-coronary causes may be seen (See My Comment in the March 1, 2023 post for the common causes of DSI ). That said — we have cath confirmation in today's case of subtotal LMain occlusion (which is consistent with the findings in ECG #1).
- It is worth taking a moment to compare lead-by-lead what the changes are that occur in the form of ST elevation and ST depression between ECG #1 and ECG #2.
- As per Dr. Nossen — this sudden deterioration in the patient's clinical condition, in association with the ST-T wave changes now seen in ECG #2 — almost certainly indicates evolution to complete LMain occlusion (even though cardiac catheterization done a short time later showed subtotal but not complete occlusion).
- PEARL: As noted in the 1st sentence of the Table in Figure-1 — a major reason why the limited data that we have on what the ECG "looks like" with acute LMain occlusion is so varied — is that we never know for certain what the state of the artery was at the time the ECG was recorded (which may be different than the state of the artery when the catheterization was done).
- In particular — Isn't it interesting how the ST-T wave appearance in lead aVR is totally reversed from the marked ST elevation seen with DSI (in ECG #1) — to marked ST depression once LMain narrowing becomes complete (in ECG #2). No wonder the limited data we have shows such variation in the ST-T wave appearance of lead aVR with LMain "occlusion".
-labeled-USE.png) |
| Figure-2: Comparison between the first 2 ECGs in today's case. RED arrows are placed at the J-point for judging ST elevation; BLUE arrows at the point for ST depression. |
- Today's case adds the extra feature of Shark Fin ST elevation on an already widened QRS complex (because of the RBBB/LAHB). That said — recognition of marked ST elevation or depression remains easy once the J-point is identified (colored arrows in Figure-2).
- In the June 11, 2018 post —
- In the October 4, 2019 post —
- In the November 22, 2019 post —
- in the January, 24, 2020 post —
- in the February 16, 2020 post —
- in the April 25, 2020 post —
- in the May 19, 2020 post —
- in the September 27, 2023 post —
- in the May 3, 2024 post —
- And — this case of a Shark-Fin-like mimic (My Comment in the August 23, 2024 post).






.png)

.png)
.png)
.png)







-USE-labeled.png)