Abstract
Background
Precautionary Allergen (“may contain”) Labelling (PAL) is used by industry to communicate potential risk to food-allergic individuals posed by unintended allergen presence (UAP). In 2014, the World Allergy Organization (WAO) highlighted that PAL use was increasing, but often applied inconsistently and without regulation — which reduces its usefulness to consumers with food allergy and those purchasing food for them. WAO proposed the need for a regulated, international framework to underpin application of PAL. In 2019, the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations convened an expert consultation to address the issue of PAL, the outputs of which are now being considered by the Codex Committee on Food Labelling (CCFL).
Objectives
To summarise the latest data to inform the application of PAL in a more systematic way, for implementation into global food standards.
Methods
A non-systematic review of issues surrounding precautionary labelling and food allergens in pre-packaged products.
Results
Approximately, 100 countries around the world have legislation on the declaration of allergenic ingredients. Just a few have legislation on UAP. Given the risks that UAP entails, non-regulated PAL creates inconvenience in real life due to its unequal, difficult interpretation by patients. The attempts made so far to rationalize PAL present lights and shadows.
Conclusions
At a time when CCFL is considering the results of the FAO/WHO Expert Consultation 2020–2023, we summarise the prospects to develop an effective and homogeneous legislation at a global level, and the areas of uncertainty that might hinder international agreement on a regulated framework for PAL of food allergens.
Keywords: Food hypersensitivity, Food labelling, Food labelling legislation and jurisprudence, Precautionary allergen labelling, Codex, Risk management, Risk communication, Prepacked, Anaphylaxis
Introduction
Ten years ago, the World Allergy Organization (WAO) published a proposal to establish an international framework for the use of Precautionary Allergen (“may contain”) Labelling (PAL) to communicate unintended allergen presence (UAP) in foods (usually, but not limited to prepacked foods).1 At the time, it was already clear that PAL use was increasing but often applied inconsistently and without regulation, which reduced its usefulness to consumers with food allergy and those purchasing food for them.2
In 2019, the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations convened an expert consultation to address the issue,3 the outputs of which are now being considered by the Codex Committee on Food Labelling (CCFL) for implementation into global food standards. We summarise here the latest data to inform PAL and review the FAO/WHO Expert Consultation recommendations. We also identify areas of uncertainty which might hinder international agreement on a regulated framework for precautionary labelling of food allergens. A definition of key terms and concepts used throughout the paper appears in Box 1.
Box 1. Glossary of relevant terms.
|
Unintended Allergen Presence
(UAP) Unintended allergen presence refers to the presence of allergens in foods that are not intentionally added, and therefore not listed as an ingredient. |
|
Precautionary Allergen Labelling
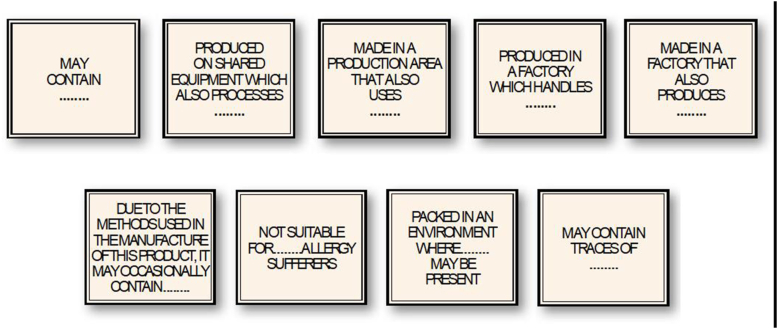
(PAL) These are precautionary statements used to inform consumers about potential UAP in foods, particularly prepacked foods. The wording used on PAL statements can vary widely, and include: “may contain”, “may contain traces of X″, or “packed in an environment where X may be present”. In contrast to consumer perceptions, the wording used does not reflect the risk of UAP and thus potential for reaction. |
|
Cross-
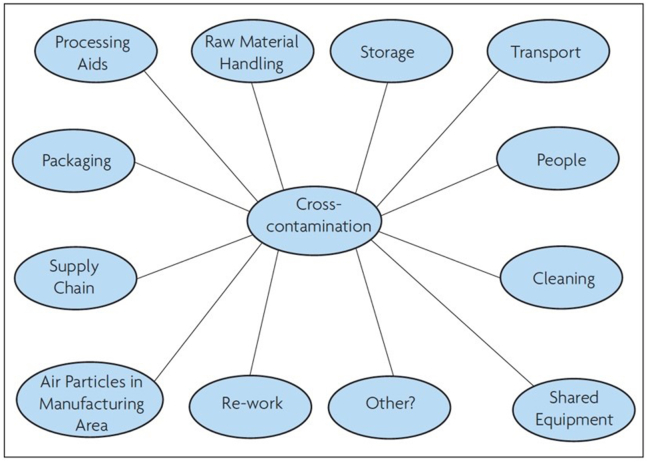
Contact Cross-contamination is the unintended transfer of food allergen into a food product, often occurring due to the use of shared equipment or facilities in the supply chain. Potential sources of cross contactinclude: handling of raw materials, storage, transport, processing aids, re-work, cleaning, shared equipment, packaging and through personnel. |
|
Risk Assessment
(RA) Risk assessment is the process of identifying and evaluating the possibility and predicted amount of UAP in food products. Ideally, RA should be standardised and regulated when used to inform the need for PAL. |
Alt-text: Box 1
Food allergy – the patient experience
The gold-standard for establishing the prevalence of food allergy is a longitudinal population-based study in a defined birth cohort, evaluating the presence of FA through double-blind, placebo-controlled oral food challenges (OFC). However, there are considerable challenges and costs involved. Thus, many studies use surrogate measures of clinical reactivity (rather than OFC), such as the presence of IgE-antibody to a food allergen with or without a recent clinical history suggestive of reaction. The use of surrogate measures can overestimate prevalence by over ten-fold.4 In children, FA prevalence has been estimated to be between 0.1% and 9.3% with large geographical variations, which suggests a larger disease burden in the developed world.5 The prevalence of food allergies has been reported at 1.5% in Hong Kong, 0.87% in Russia, 0.21% in mainland China, and 0.14% in rural India.6 Data are more limited for adults. A population-based survey of US adults estimated a prevalence for FA of 10.8%, of which around half were adult-onset.7 In the United Kingdom, FA prevalence in adults has been estimated to be between 1.0 and 1.8% (population-based cohort of ∼7 million individuals)8 and 5.7% for IgE-mediated FA in a community survey, some of whom underwent confirmatory OFC.9
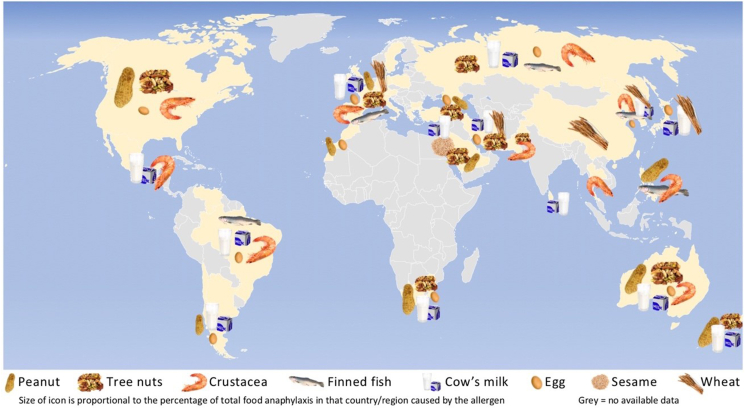
In terms of the most common triggers for food-induced allergic reactions, a systematic review recently evaluated the most common foods causing anaphylaxis presenting to Emergency Departments globally (Fig. 1).10 As reported by the authors, the most common triggers generally corresponded to stated “priority” allergens for any given region (see Section 2).
Fig. 1.
Predominant causes of food anaphylaxis globally. Reproduced with permission from Baseggio Conrado et al.10
Living with FA, or being a caregiver for an affected individual, has a major impact on socialisation and household finances. Food allergies impact several domains in quality-of-life, including attendance at daycare/school/college; social interactions, including birthday parties; family relationships; finances; shopping for safe foods; eating out and travelling; and mental health.11 This results in a decreased quality-of-life, primarily driven by the impact on social interactions and dietary diversity.12 Children/young people and adults report increased stress and anxiety relating to fear over accidental reactions (which can exacerbate social isolation); concern about one's confidence and ability to deal with reactions (self-efficacy); adverse influence on interpersonal and social relationships; restrictions on career options; and fears for the future. Food-allergic consumers move between balanced vigilance and appropriate dietary avoidance (which promotes effective coping strategies) and periods of hypo/hypervigilance, putting physical health or psychological wellbeing at risk.13 Ambiguity over the interpretation of allergen food labels is a key driver of the impairment in quality-of-life reported by food-allergic individuals and their carers.2,14
Current legislation for food allergen declaration
In order to promote safe food choices, relevant information must be communicated to consumers in a transparent and accessible manner. In addition, food-allergic consumers must be able to trust the information provided.15
The Codex Alimentarius (often abbreviated to “Codex”) is a collection of internationally adopted food standards and related texts presented in a harmonized document, overseen by WHO and FAO. The Codex standards aim to protect consumer health and ensure fair practices in international food trade, and include provisions in respect of food hygiene, food additives, food contaminants, labelling and presentation, import/export inspection and certification. Since 1985, Codex has included food allergens, with the General Standard for the Labelling of Prepackaged Foods (GSLPF)16 requiring mandatory declaration of 8 “priority” allergens.
-
•
Cereals containing gluten; ie, wheat, rye, barley, oats, spelt or their hybridised strains and products of these;
-
•
Crustacea and products of these;
-
•
Eggs and egg products;
-
•
Fish and fish products;
-
•
Peanuts, soybeans and products of these;
-
•
Milk and milk products (lactose included);
-
•
Tree nuts and nut products; and
-
•
Sulphite in concentrations of 10 mg/kg or more.
Strictly speaking, sulphite is not an allergen but a chemical preservative that can elicit respiratory symptoms consistent with an allergic reaction. For this reason, sulphites were not included as an allergen in the recent FAO/WHO Expert review.17 CCFL is currently revising the GSLPF based on the recommendations of the FAO/WHO Expert review, and it is likely that in the future, oats will be considered separately from gluten-containing cereals. Soy and peanut will be considered as separate allergens, and tree nuts will need to be specified by name.
Currently, almost 100 countries have legislation requiring mandatory declaration when an allergen is intentionally present as an ingredient. The Codex standards have been adopted by most countries, either by reference to Codex itself or its inclusion in local legislation (see Table 1). Many countries have modified the list of “priority” allergens to meet local needs.
Table 1.
Legislation with respect to mandatory disclosure of allergens in prepacked foods across different countries.
| Mandatory allergen
declaration when present as an ingredient |
Precautionary allergen
labelling |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | Other gluten-containing cereals | Egg | Milk | Peanut | Tree nuts | Soy | Fish | Crustacean | Mollusca | Celery | Mustard | Sesame | Lupin | Sulphur dioxide | Other | Use regulated? | Details over PAL use | |
| Codex | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | not currently | ||||||||
| Argentina | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓a | YES | PAL prohibited, but “contains …” permitted as an alternative if UAP risk can be justified | |||||
| Australia/New Zealand | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Guidance available | ||||
| Brazil | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓a | |||||||
| Canada | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Guidance available. PAL must not be misleading | ||||
| CARICOMf | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Central Americag | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Costa Rica, Chile, Mexico and Venezuela have legislation regarding UAP. | |||||||
| Chile | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| China | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Legislation currently being drafted | ||||||||
| European Union (EU)h | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Guidance available in some member states, sometimes based on thresholds.e | ||
| Gulf statesi | ✓ | ✓ | ✓ | ✓ | ✓ | Walnut | ✓ | ✓ | ✓ | Clams | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| India | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Japan | ✓ | ✓ | ✓ | ✓ | ✓ | b | b | b | ✓b | b | ✓b | YES | UAP >10 ppm must be declared as ingredient | |||||
| Malaysia | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Mexico | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Singapore | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| South Africa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | YES | Manufacturers must declare PAL is needed, but this is not guided by a threshold. | ||||||
| South Korea | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓c | ✓c | ✓c | Official guidance available | ||||||||
| Switzerland | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | YES | PAL can only be use for non-ingredients >100 ppm for gluten or >1000 ppm for other allergens | |
| Thailand | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| UK | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Official guidance available | ||
| USA | ✓ | ✓ | ✓ | ✓ | ✓d | ✓ | ✓ | ✓ | ✓ | |||||||||
Table updated from Allen et al.
Local legislation also requires mandatory disclosure of tartrazine.
Local legislation requires mandatory disclosure of eggs, milk, wheat, buckwheat, peanut, walnut, shrimp and crab (rather than crustacea). Disclosure is recommended (but not required) for the following: almond, abalone, squid, salmon roe, oranges, cashew, kiwifruit, beef, sesame, salmon, mackerel, soybean, chicken, banana, pork, macadamia nut, peach, yam, apple and gelatin.
Local legislation requires mandatory disclosure of eggs (confined to those from poultry), milk, buckwheat, peanuts, soybeans, wheat, mackerel, crab, shrimp, pork, peach, tomato, sulphites, walnuts, chicken, beef, squid, clams (including oyster, abalone, and mussels), pine nut. There are no allergens for which labelling is optional.
Tree nuts in the USA include a range of native nuts not included, for example, under EU legislation e.g. Beech, Butternut, Chestnut, Coconut, Ginko nut, Hickory nut, Lychee, Shea nut.
Since January 1, 2024, the Netherlands has a new policy for cross contactand PAL, including RfDs. Companies have a transition period of two years to comply with the new policy, so until January 1, 2026.92
CARICOM is an organization of Caribbean countries.
Central American Technical Regulation has been adopted by Costa Rica, Guatemala, Honduras, El Salvador and Nicaragua.
Countries adopting EU legislation for allergen declaration are EU member states and Iceland, Lichtenstein, Norway, Macedonia, Switzerland.
Members of the Gulf Cooperation Council are: United Arab Emirates, Bahrain, Saudi Arabia, Oman, Qatar, Kuwait
In Europe, most countries are aligned with legislation from the European Union (EU), which mandates the declaration of 14 allergens (the Codex-8, with peanut and soya named separately, plus celery, mustard, sesame, lupine, molluscs).18 The most recent relevant legislation is EU Regulation 1169/2011,18 commonly known as Food Information to Consumers (FIC), which requires mandatory declaration for these 14 allergens for both prepacked and non-prepacked foods (including those sold by catering outlets such as restaurants). FIC also stipulates how ingredients should be listed in terms of font size on labels. “Contains” statements are prohibited; instead, allergens must be declared within the ingredients list, and emphasised (e.g. in bold print). Specific disclosure of individual gluten-containing cereals and tree nuts is required.
Japan, which was the first country to regulate both intentional and unintended allergen presence (UAP), uses a localised list of allergens, divided into those for which disclosure is mandatory (wheat, buckwheat, egg, milk, peanut, shrimp, crab and walnut) and allergens where disclosure is recommended but not required (almond, abalone, squid, salmon roe, oranges, cashew, kiwifruit, beef, sesame, salmon, mackerel, soybean, chicken, banana, pork, macadamia nut, peach, yam, apple and gelatin).19 A similar approach has been adopted in South Korea, although the list of allergens is different.20 The US has implemented mandatory allergen declaration through the Food Allergen Labelling and Consumer Protection Act 2004 (FALCPA).21 FALCPA includes the same allergens as Codex, with the notable exception that wheat is the only named cereal (other gluten-containing grains are not included). Subsequently, the 2021 Food Allergy Safety, Treatment, Education, and Research (FASTER) Act amended FALCPA to add sesame as an allergen.22 For tree nuts, the specific nut must be declared; for crustacea and fish, the species must be declared.23 “Gluten-free” is a voluntary claim; if used on a food label, gluten must not exceed 20 parts per million (ppm). Priority allergens in Canada include the Codex-8, but also molluscs and mustard. In both US and Canada, priority allergens must be declared in the ingredients list, or optionally using a separate “Contains” statement for priority allergens23,24 — something prohibited in the European Union.18 A “contains statement” is mandatory for priority allergens in Australia and New Zealand.25
Precautionary allergen labelling
Food production has become increasingly complex, involving multiple supply chains across many countries to try and drive efficiencies and lower costs to the consumer. Despite adherence to “Good Manufacturing Practices” (GMP), allergens may be present because of unintentional cross-contact (also referred to as cross-contamination) between foods either during harvesting, storage, transport or processing (Fig. 2). The Codex standard is widely interpreted as being applicable only to intentionally-added components – and not to allergens that may inadvertently be added during the supply chain or production (ie, UAP). In many countries, this distinction is made explicit. For example, in Australia/New Zealand, the European Union and United Kingdom, local regulations make it explicit that labelling applies only to allergens (or their derivatives) present as a result of intentional use as an ingredient, and not due to UAP.
Fig. 2.
Potential sources of UAP due to cross-contact (reproduced with permission)1.
Many food businesses mitigate against the risk of UAP using PAL, although this does not replace the need for compliance with GMP and risk management protocols such as Hazard Analysis Critical Control Point (HACCP). PAL emerged in the 1980s as a risk-communication measure to allow food businesses to provide information to allergic consumers that a product might pose risk to them (ie, trigger an allergic reaction) due to allergen cross-contact or cross-contamination, because of production on shared equipment or allergens being present within the same facility. Examples of PAL statements are shown in Fig. 3.26
Fig. 3.
Examples of precautionary allergen labels found on prepacked foods. Reproduced with permission26.
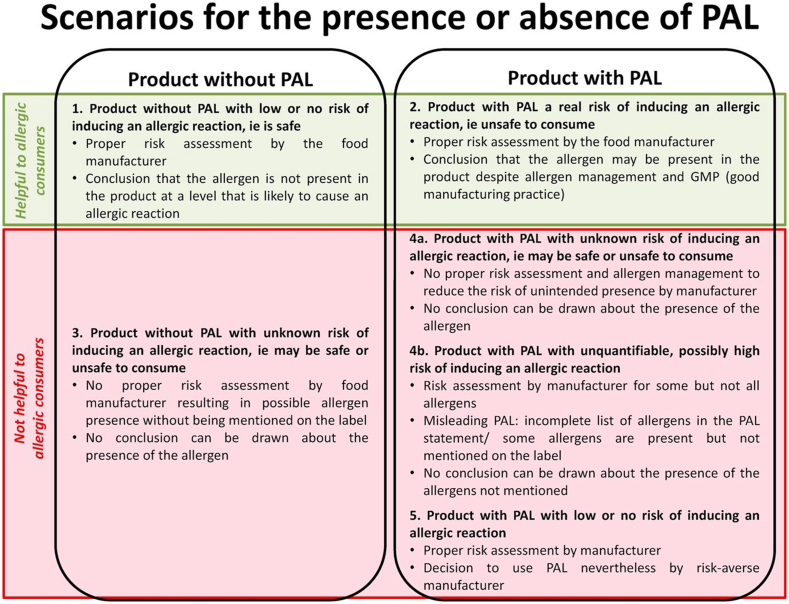
In almost all countries, PAL statements are not regulated or standardised,26,27 although guidance in some countries (United States, Canada, European Union, and United Kingdom) states that PAL cannot be misleading. This has resulted in very inconsistent application by food businesses, with consumers often unaware of the reason why a PAL is present (or absent), and whether a risk assessment has been conducted. (Fig. 4). Consumers frequently perceive that PAL are used by manufacturers as a “safety net” to convey an unspecified risk of possible cross contact, rather than actual risk.2
Fig. 4.
Scenarios for the presence or absence of precautionary allergen labelling (PAL)2,27.
In 2014, WAO published a summary of the inconsistencies in PAL application, and the legislative “grey zone” that exists.1 A few countries stipulate rules over the use of PAL (see Table 1), although these rules are not always consistent nor helpful in communicating actual risk to consumers. In the European Union, PAL are not regulated; however, the Czech Republic has instituted legislation relating to UAP since 2018, while other member states (eg, Belgium, the Netherlands) have instituted local guidelines and intent-to-legislate notices to establish “action levels” for PAL – although there have been significant variations in the action levels proposed,27 something which increases the potential for inconsistent application of PAL. Further confusion arises from the different regulatory approaches to the use of “Contains …” statements for declaring intentionally-added ingredients that are allergens (voluntary in some countries such as the United States and Canada, mandatory in others eg, Australia, prohibited in the European Union).
PAL is now used for the majority of supermarket products in Australia (65%, 2011),28 Canada (72%, 2021;29 67%, 202230), United Kingdom (55%, 2014),31 Latin America (33–63%, 2020),32 and a large percentage in the United States (17% across all food categories, >50% for candy and cookies, 2009).33 The latter is almost certainly an underestimate, reflecting the frequent use of “minor ingredient labelling” in the United States, a practice now appearing in other countries too. This scenario occurs when manufacturers declare an unintended allergen as an actual ingredient (appearing as 1 of the last 3 ingredients on the label), perhaps to serve as a stronger deterrent than PAL.34 However, this practice has seen a resurgence with the intentional addition of small amounts of sesame to food products by manufacturers to circumvent the need to risk-assess and manage potential UAP due to sesame — something now required in the United States due to sesame being added as a priority allergy in the FASTER Act.35
The high proportion of products with PAL increases the perception that PAL are applied indiscriminately.2,26 The use of multiple PAL statements, each with different wording, adds to this perception. For example, a 2006 US survey of supermarket products reported 25 different PAL statements on 3442 different products,33 while a similar survey in Latin America found 33 different types of PAL statement on food products.32 Shopping for a food-allergic person takes longer and costs more, which has obvious implications on consumer choice.36 This is likely to cause an even higher burden in families with low or no income, exacerbating existing socioeconomic disparities in allergy care provision. Social food schemes (such as food kitchens) can struggle to provide safe food options for people with food allergies, due to how ingredients are sourced.
Do foods with PAL cause allergic reactions?
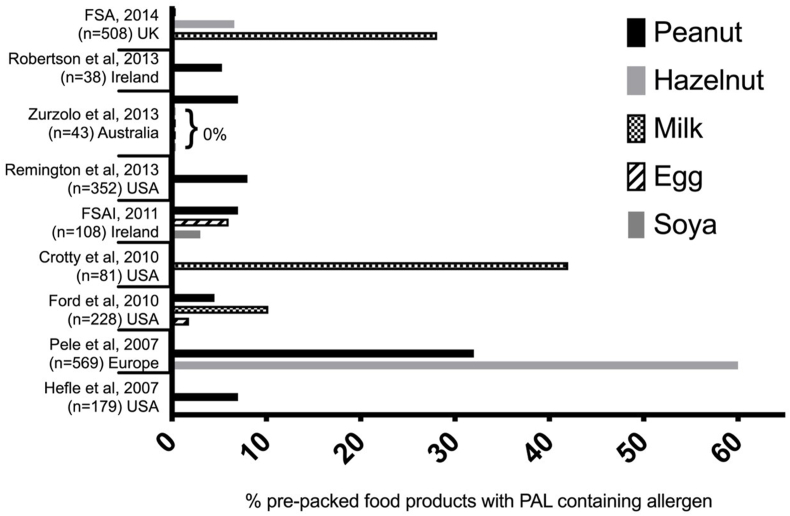
A number of studies have evaluated whether prepacked foods with or without PAL actually contain a priority allergen not listed in the ingredients. These data are summarised in Fig. 5. For peanut, the majority of prepacked foods with PAL do not contain detectable amounts of peanut, particularly for non-confectionery items.37 This holds true for other allergens such as cow's milk, egg, hazelnut, or soya in those countries where data are available, although there are some important exceptions, particularly with chocolate products where UAP to cow's milk is common.38 These data are not entirely surprising. UAP can occur in 2 forms: particulate and non-particulate contamination. Non-particulate allergens are ingredients such as powders or liquids, which are usually distributed relatively uniformly.39 In contrast, particulate contamination (see Fig. 6) is infrequent and results in non-uniform distribution in the final food product. This poses significant difficulties with risk assessment, and results in the common scenario where most products with PAL do not have UAP — because the risk and distribution of particulate contamination is sporadic.
Fig. 5.
Summary of studies reporting proportion of prepacked foods with PAL to priority allergens that actually contained the allergen on biochemical analysis. In all these studies, contamination has only been detected in "snack" foods (chocolates, biscuits/cookies, muesli/trail bars, baked snacks, confectionery, ice cream).37
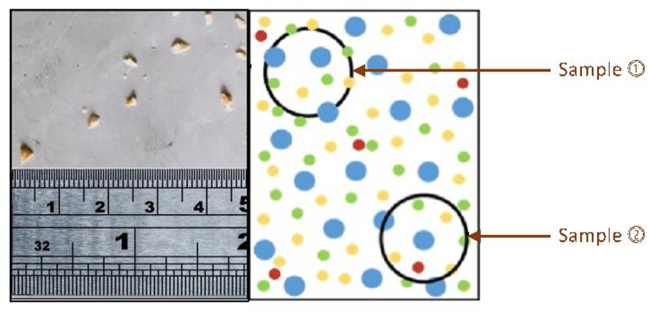
Fig. 6.
Particulate contamination. Allergen fragments (represented as red dots in the right panel) are not uniformly distributed in the food product, and can be sporadic – affecting some batches but not others. This creates difficulties during risk assessment, as 1 sample may not contain any allergen (Sample 1) while another (Sample 2) does. This results in the common scenario where products infrequently contain UAP, but are labelled appropriately with PAL.
These same surveys have also shown that sometimes, prepacked foods without PAL can contain undeclared allergen.40, 41, 42, 43, 44 For example, Pele et al reported an analysis of 544 cookie biscuits and chocolates sourced from 10 European countries: peanut was detected in 108/333 (32%) of prepacked foods with PAL, but also in 52/211 (25%) of products without PAL.44 Hazelnut was detected in 76% of chocolates with PAL, and 50% of chocolates without PAL. These data show that PAL does not always indicate actual UAP, but nor does absence of PAL imply no or even low risk of contamination. Furthermore, the wording of the PAL statements bear no relationship with the risk posed and can mislead consumers. These studies have highlighted that products with PAL phrases which may be interpreted as conveying a lower risk of UAP (eg, “made in an environment … ”) (see Table 2) frequently UAP of a magnitude similar or even greater than those labelled “may contain … ”.43,44
Table 2.
Summary of consumer surveys reporting the proportion of individuals who would always avoid purchasing products with Precautionary Allergen Labelling (PAL) due to food allergies, and how this is impacted upon by the phraseology used.
| PAL wording | “May contain” | “May contain traces” | “Manufactured in a facility that also processes …” | “Not suitable for …” | Reference |
|---|---|---|---|---|---|
| UK (n = 184) | 80% avoid | 60% avoid | 40% avoid | n/a | Noimark et al. 2009 |
| Canada (n = 127) | 56% avoid | 47% avoid | 40% avoid | 80% avoid | Ben-Shoshan et al., 2012 |
| Australia (n = 246) | 75% avoid | 45% avoid | 35% avoid | n/a | Zurzolo et al., 2013 |
| Netherlands (n = 179) | 64% avoid | 43% avoid | 36% avoid | n/a | DunnGalvin et al., 2015 |
| Ireland (n = 87) | 67% avoid | 59% avoid | 49% avoid | n/a | |
| UK (n = 161) | 70% avoid | 61% avoid | 53% avoid | 81% avoid | |
| Germany (n = 474) | 70% avoid | 45% avoid | 39% avoid | 82% avoid | |
| USA (n = 5507) | ∼90% avoid | n/a | 59% avoid | n/a | Marchisotto et al., 2017 |
| Canada (n = 1177) | 77% avoid | n/a | 64% avoid | n/a | |
| USA (n = 2729) | 81% avoid | 86% avoid | 50–80% avoid | n/aa | Gupta et al., 2021 |
| Netherlands (n = 42) | ∼90% avoid | ∼70% avoid | ∼30% avoid | n/a | Holleman et al., 2021 |
Data not available, but this term (“not suitable for”) was found to be most preferred by consumers (29.3%), followed by “May contain allergen” (22.1%)
Remington et al. undertook an analysis of how often foods labelled with PAL could cause a reaction in allergic consumers, using data from the United Kingdom. While the vast majority of foods did not contain UAP, those that did would have caused a reaction in >1% of the allergic population, and for some foods (chocolate), the risk could have been up to 50%.45
However, few studies have investigated how common accidental reactions are in real life, due to UAP. Sheth et al reported 651 food-allergic patients in a Canadian registry who experienced an allergic reaction due to inadvertent allergen exposure; 37% attributed their reaction to a failure to read or heed PAL, and one-third of these thought their reaction was due to UAP not declared with PAL.46 In Australia, 6.7% (58/864) of responders to a survey run through a patient support organisation reported anaphylaxis due to undisclosed allergen in prepacked foods, although PAL to the allergen was present in 81% of cases.47 A retrospective survey of 100 children in Switzerland who regularly consumed foods with PAL found that 18% reported a previous reaction to a food with PAL (the most common products were chocolates, cookies and cakes). Reported reactions were all mild (skin and/or gastrointestinal symptoms).48
In a prospective study in 157 adults with physician-confirmed food allergy in the Netherlands, 153 reactions were reported by 73 patients;49 62 (41%) of these were due to prepacked foods; in one-third of cases, the suspected allergen(s) were not mentioned either as an ingredient or on PAL. Allergen analysis was conducted in 51 samples (67% were prepacked foods): UAP was detected in 19, of which 10 (53%) did not have a corresponding PAL statement.50
Irrespective, the surveys also indicate that aside from “risk anomalies” (for example, the production of dark chocolate on the same line as milk chocolate results in a relatively high frequency of UAP to milk38), the amounts of potential UAP would not be expected to cause anything other than mild allergic symptoms in the vast majority of food-allergic individuals.51,52 In support of this observation, low-level milk contamination in foods (corresponding to an ingestion of 0.5–2 mg of milk protein) did not cause reactions in a cohort of 29 milk-allergic children with a history of prior anaphylaxis.53 Similarly, there are very few reports in the literature of potentially life-threatening reactions due to UAP,54 despite evidence suggesting that many or even most food-allergic individuals eat foods with PAL (see Table 2). There are 2 reports from the United States where it was suggested that consumption of foods with PAL caused a fatal reaction: 1 over 20 years ago following consumption of cake, and another around 10 years ago in a peanut-allergic teenager who consumed a snack bar (of dried fruits, nuts and seeds) which did not contain peanut as an ingredient but did have a PAL to peanut.55 There are no reports of fatal reaction due to UAP in a prepacked food with PAL in the UK Fatal Anaphylaxis Register which has collected data for over 25 years, although there has been at least 1 fatality due to consumption of a prepacked-for-direct-sale vegan wrap which contained undeclared cow's milk but no PAL.
How do food-allergic consumers, and those advising them, interpret PAL?
Given the proliferation of products with PAL, it is not surprising that surveys report that allergic consumers (or those purchasing food for them) often ignore PAL (see Table 2). There is a common theme: the public often assumes that the wording of the PAL statement conveys useful information as to the magnitude of potential UAP, that an item with a "may contain" PAL poses a greater risk than other PAL statements that may be perceived as more neutral (such as “manufactured in a facility which also processes X"). Consumers rely on instinct to make decisions whether or not to consume a product with PAL.56
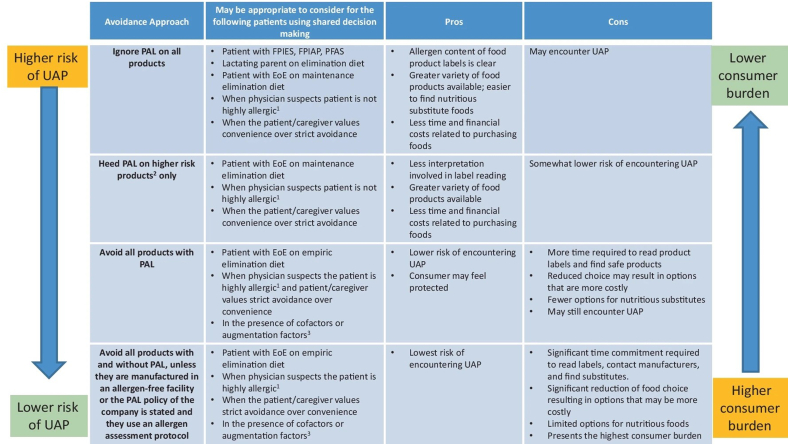
Clinicians may not discuss how to interpret PAL with patients, nor is there a consensus amongst as to what advice to provide. Turner et al surveyed 239 healthcare professionals in the United Kingdom providing dietary advice to allergic consumers or their caregivers: 38% recommended complete avoidance of foods with PAL to nuts (but no nut listed in the ingredients), while 22% advised no avoidance was necessary, with the remainder taking a permissive approach to PAL depending on the patient's health and access to rescue medication.57 Clearly, there is variability in the advice healthcare professionals provide with respect to PAL, ranging from strict allergen avoidance to ignoring PAL altogether (Fig. 7).58
Fig. 7.
Different management considerations with respect to PAL. Reproduced from Schaible et al. 2024.58,1 “Highly allergic” refers to a significant systemic reaction to a low dose exposure. 2Food items reported to have a higher risk of UAP include chocolate and chocolate-based foods, nutrition/meal bars, granola/muesli bars, cookies/biscuits, confectionery and ice cream. 3Cofactors (such as exercise, infections, fever, non-steroidal anti-inflammatory, alcohol, stress) can result in the occurrence of more severe allergic reactions in some people
Both patients/families and clinicians are confused as to whether PAL are required by law. Marchisotto et al surveyed 6684 participants in North America (22% with a food allergy themselves, 84% caregivers): 46% were either unsure or incorrectly believed that PAL is required by law, and 37% thought the wording used for PAL reflected how much UAP might be present.59 In a survey of healthcare professionals in the United Kingdom and Australia, 32% wrongly believed that PAL use was subject to a standardised risk assessment and 13% believed that PAL was regulated by law.60 Only 50% considered that PAL statements such as “may contain X″ or “X may be present” indicated a real risk of UAP. There was a strong consensus for the introduction of a single and simple PAL statement, something also reported by allergists in Canada.61 Some regulators now recommend specific phrases: both Health Canada62 and the UK Food Standards Agency63 recommend the simple/unequivocal phrase “May contain X” without further qualification.
Although official guidance is welcome, our conclusion from 2013 remains valid today: that “the current situation [with respect to PAL] does not benefit either the allergic consumer or food manufacturers, who are potentially liable for an allergic reaction resulting from cross-contamination”.1
Attempts to rationalize the use of PAL
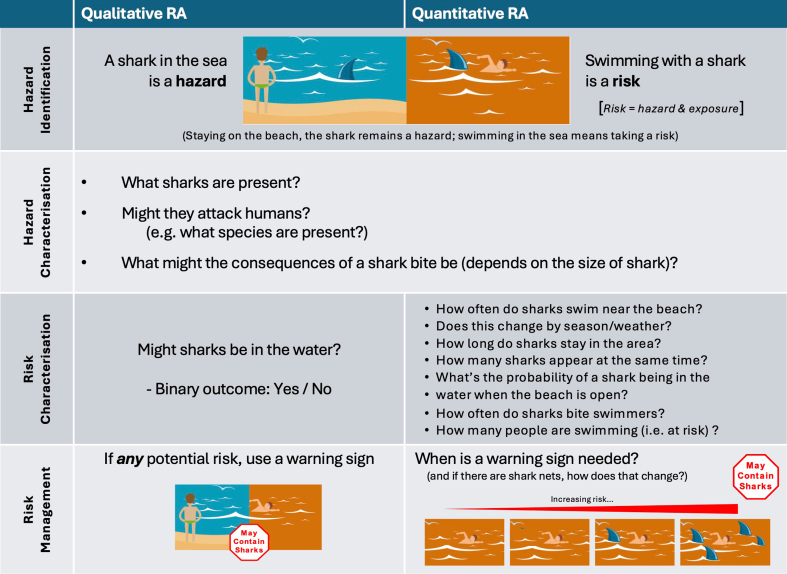
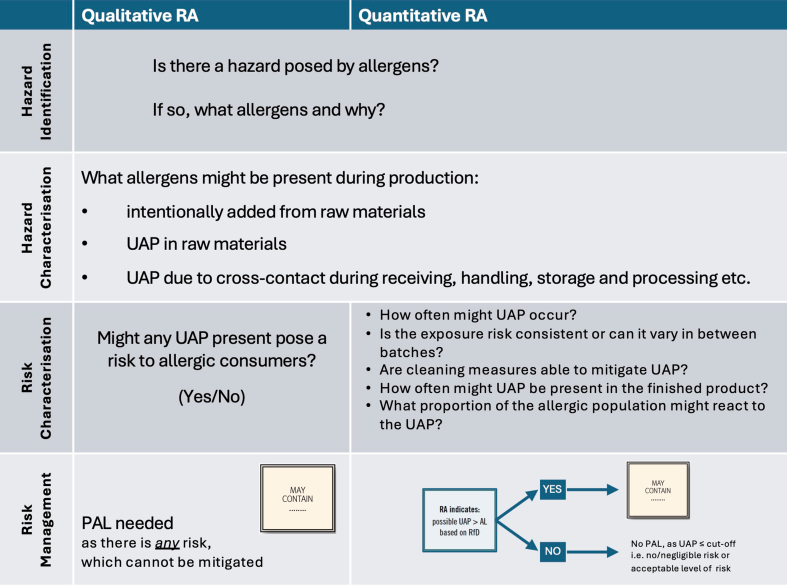
Madsen et al reviewed different approaches to risk assessment in food allergy.64 Risk assessment processes applied to UAP involves 4 distinct steps: identification of the hazard, characterisation of the hazard, characterisation of the risk, and then assessment of risk mitigation measures. The difference between a hazard and a risk is shown in Fig. 8. Risk considers both the hazard, and the likelihood of exposure to that hazard causing an adverse outcome. This can be assessed in a qualitative manner, or a quantitative risk assessment applied (Fig. 8).
Fig. 8.
Infographic to explain the difference between a qualitative and quantitative risk assessment. Graphics adapted from EFSA Hazard vs Risk infographic.
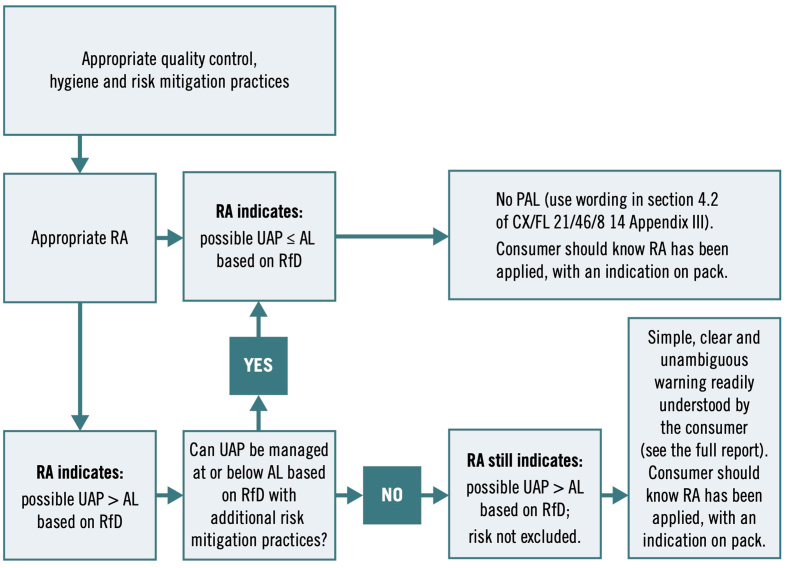
Quantitative or qualitative RAs are structured science-based processes that estimate the probability and severity of illness from consuming food containing biological, chemical or physical contaminants. They also identify where in the production chain controls and interventions will have the greatest impact on reducing risk. With respect to UAP, a qualitative RA essentially involves answering a YES/NO as to whether there is a risk of any UAP, while quantitative RA provides a more pragmatic assessment of the degree of risk posed by UAP to allergic individuals, to guide whether a PAL is needed (see Fig. 9).
Fig. 9.
Risk assessment process applied to the evaluation of UAP.
Using a set concentration to inform PAL: Japan, Switzerland, and the Czech Republic
The first country to define a labelling threshold was Japan. Since 2002, legislation has required the declaration of specific food allergens when present at a concentration greater than 10 ppm (μg protein/g food) soluble allergen protein, irrespective of whether the allergen is present due to UAP or not.65 The 10 ppm threshold was established on the basis of analytical limits of detection for immunoassays to the major allergens. If UAP exists below 10 ppm, then a “may contain” phrase is prohibited although alternative PAL statements can be used. UAP greater than 10 ppm must be declared as an ingredient. In 2006, further criteria were integrated into official guidelines to standardise allergen detection.66 Local clinicians report that this approach has been successful, with no significant reactions reported due to UAP in prepacked foods since implementation (M. Ebisawa, personal communication).
Switzerland also established action levels for PAL in 2005. PAL must be used for UAP which exceeds 100 ppm for gluten or 1000 ppm (total allergen, not protein) for other allergens; PAL is allowed below these action levels, but is not mandatory.67 This equates to 25 mg in a 100 g serving for non-gluten allergens (assuming a protein content of 25%, as is the case for peanut) – an amount predicted to cause objective symptoms in around 20% of peanut-allergic individuals. Unfortunately, there are no published data assessing the impact of this threshold on the incidence of allergic reactions due to UAP, although at least 1 Swiss specialist has expressed concerns that the current threshold is not sufficiently protective.68
The Czech Republic implemented legislation relating to PAL in 2018.69 PAL are not required below amounts which vary from 1 to 2.5 ppm for most priority allergens (20 ppm for gluten); these cut-offs were based on analytical capability, although these cut-offs are significantly lower than those applied in Japan, also on the basis of analytical capability. A maximum level for UAP was also set (at 10–50 ppm, depending on the allergen), above which UAP is considered to be a failure of risk management and affected food products are prohibited from sale.
VITAL® program
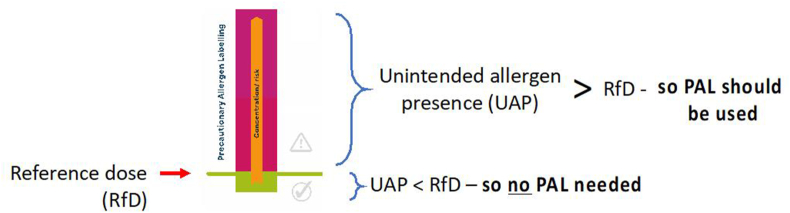
A quantitative, risk-based approach to PAL was first proposed in 2005 by the Allergen Bureau of Australia and New Zealand (a not-for-profit organisation representing national and multinational food businesses). In response to concerns over the widespread use of PAL to mitigate against risk of UAP from shared production lines, the Allergen Bureau developed VITAL® (Voluntary Incidental Trace Allergen Labelling), a voluntary, standardised allergen risk assessment process for the food industry. VITAL® provided guidance and risk assessment tools to allow food businesses to make risk-based management decisions regarding the need for PAL statements, based on a cut-off which is referred to as an “action level”. The concept is shown in Fig. 10.
Fig. 10.
Concept underpinning action levels to determine the need for PAL. If the degree of unintended allergen presence (UAP) is greater than the “reference dose” (RfD) for that allergen, then a PAL statement should be applied.
The first iteration of the VITAL® scheme (VITAL 1.0) was released in 2007. This used 2 action levels (expressed as a single concentration of allergenic protein), above or below which PAL was or was not recommended.70 The action levels were based on reference doses (RfDs) derived from lowest observed adverse effect levels (LOAELs) – the lowest ingested dose that would cause an objective allergic reaction — based on published data from OFCs analysed by the University of Nebraska Food Allergy Research and Resource Program (FARRP).71 This approach was used in a US Food & Drug Administration (FDA) Working Group report on Allergen Thresholds.72 A ten-fold “uncertainty factor” was also applied, as is usually the case with toxicological risk assessment when relying on data from animal models.
The ppm cut-offs were derived on the basis of a food serving of 5 g (1 Australian teaspoon) as the reference quantity: the rationale was that the resulting action level would reflect the lowest amount of allergen in a small mouthful of food. However, there was criticism over the use of a fixed food portion size: people do not react to concentrations but an actual quantity of allergen, and use of a fixed portion size did not reflect this reality. Furthermore, the RfDs available at the time were not yet based on sound clinical science. This led a collaboration between the Allergen Bureau and FARRP to expand the dataset informing RfDs, which led to the VITAL® Scientific Expert Panel (VSEP) being established in 2010.
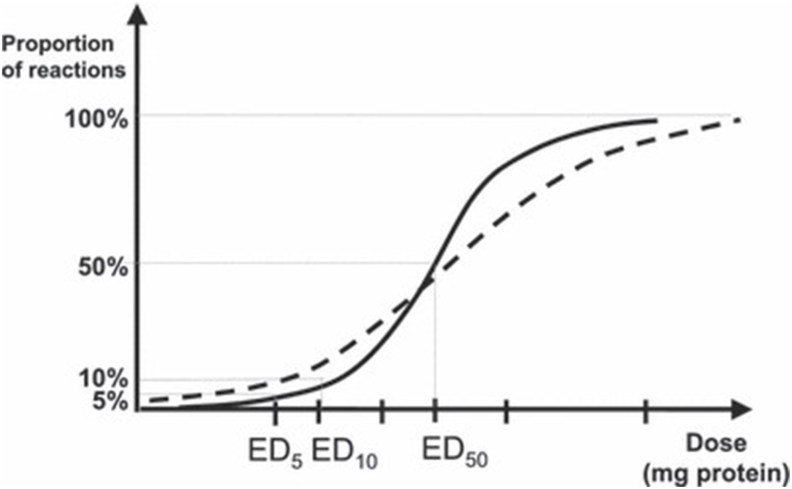
Subsequent iterations of VITAL® (VITAL-2 and VITAL-3) addressed these criticisms: statistical dose-distribution modelling was applied to a more extensive dataset of OFC data to derive RfDs; reference amounts (typical serving portions) were used to inform the potential exposure level.73 VITAL® 2.0 action levels were expressed as milligrams of total protein from allergenic foods (instead of a concentration), and where there was sufficient precision, were based on the ED01 — the amount of allergenic protein expected to trigger objective reaction in 1% of the population allergic to that specific allergen. In contrast to VITAL® 1.0, no uncertainty factor was applied, since the data were derived from OFC in humans and the uncertainty captured by the statistical modelling (Fig. 11).74 A further review in 2020 further extended the dataset, and applied a new Stacked Model Averaging approach75 to derive reference doses based on ED01 for the major allergens (Table 3, Fig. 12).76
Fig. 11.
Derivation of Eliciting Doses (EDs) for different proportions of the food-allergic population, based on OFC data. The figure shows the possible differences between dose distributions in the challenged population (solid line) and the general, food-allergic population (dashed line); this difference is captured by the statistical modelling used to estimate EDs. Reproduced from Crevel et al. with permission74.
Table 3.
Food challenge (FC) datasets used to inform the VITAL® 2.0 and 3.0 RfDs
| Allergen | 2011
recommendations |
2019
Recommendations |
FAO/WHO 2023 Expert Consultation Proposed RfD (mg protein) | Equivalent concentration (ppm) with respect to 100 g serving size | |||
|---|---|---|---|---|---|---|---|
| FC Dataset (n) | VITAL 2.0 RfD (mg protein) | FC Dataset (n) | VITAL 3.0 RfD (ED01) (mg protein) | ED05 (mg protein) | |||
| Egg | 206 | 0.03 | 431 | 0.2 | 2.3 | 2.0 | 20 |
| Hazelnut | 200 | 0.1 | 411 | 0.1 | 3.5 | 3.0 | 30 |
| Lupin | 24 | 4.0 | 25 | 2.6 | 15.3 | 10.0 | 100 |
| Milk | 344 | 0.1 | 450 | 0.2 | 2.4 | 2.0 | 20 |
| Mustard | 33 | 0.05 | 33 | 0.05 | 0.4 | 1.0 | 10 |
| Peanut | 744 | 0.2 | 1306 | 0.2 | 2.1 | 2.0 | 20 |
| Sesame | 21 | 0.2 | 40 | 0.1 | 2.7 | 2.0 | 20 |
| Shrimp | 48 | 10.0 | 75 | 25 | 280 | 200 | 2000 |
| Soy | 51 | 1.0 (soy flour) | 87 | 0.5 | 10.0 | 10.0 | 100 |
| Wheat | 40 | 1.0 | 99 | 0.7 | 6.1 | 5.0 | 50 |
| Cashew | 31 | – | 245 | 0.05 | 0.8 | 1.0 | 10 |
| Celery | 39 | – | 82 | 0.05 | 1.3 | 1.0 | 10 |
| (Fin)fish | 19 | – | 82 | 1.3 | 12.1 | 5.0 | 50 |
| Walnut | 15 | – | 74 | 0.03 | 0.8 | 1.0 | 10 |
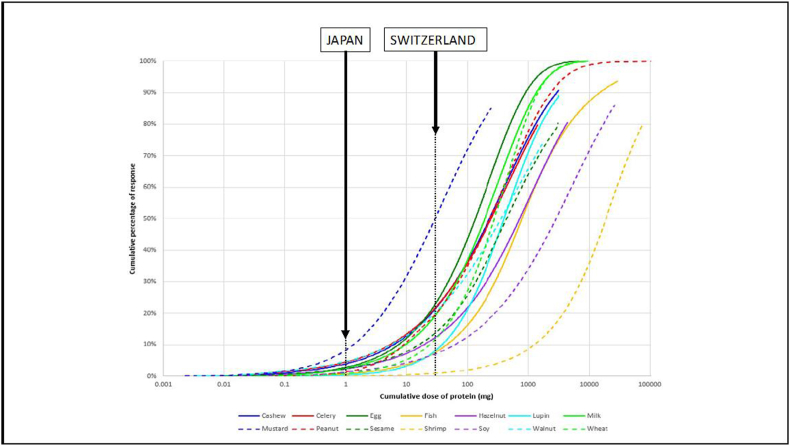
Fig. 12.
Eliciting dose curves for the EU-14 priority allergenic foods, based on cumulative dose datasets (expressed as mg total protein).75 Also depicted are the regulatory thresholds set by Japan (10 ppm [mg/kg]) and Switzerland (1000 ppm [mg/kg], assuming 25% protein content) for a 100 g serving.
Studies have sought to assess the validity of using LOAELs to derive Eliciting Doses (EDs) for different proportions of the food-allergic population. Two individual participant data (IPD) meta-analyses have evaluated intra-individual variability in reaction thresholds (reproducibility) to peanut77 and cow's milk.78 These have demonstrated that reaction thresholds are relatively stable over time intervals of up to 1–2 years, although there are limited longitudinal data to inform longer intervals and the impact of natural resolution. At a population level (where cross-sectional datasets are arguably more relevant) the data are reassuring, although individual patients may need specific personalised advice in terms of how their sensitivity may change over time. While cofactors (such as exercise, stress, medication etc) can impact on thresholds (and severity) in some individuals,79 their impact does not appear to be any greater than the inherent variability in both clinical thresholds and risk of anaphylaxis identified in the wider food-allergic population.80 This was also the view of the FAO/WHO Expert Group (see 6.4),51 although it was noted that food-dependent exercise-induced anaphylaxis (FDEIA) and possibly food-dependent non-steroidal anti-inflammatory drug-induced anaphylaxis (FDNIA)81 may be important exceptions.
Other studies have evaluated the impact of age and geographic region on differences in EDs. Allen et al reported little effect of age on estimated EDs at or below ED10 for peanut or hazelnut.82 Evaluating data from different regions, ED05 estimates for peanut from France, The Netherlands and the United States were not substantially different (2–4 mg peanut protein). However, a lower ED05 was reported for the United Kingdom, possibly due to the inclusion of more sensitive patients (from immunotherapy studies) in that country. More recent studies have reported ED05 values of 3.8 (2.4–5.7) mg in UK adults79 and 12.1 (9.6–15.2) mg peanut protein in Danish children and adult populations,52 respectively, providing greater certainty and reassurance. More recently, single-dose challenges have confirmed the validity of ED05 estimates in peanut-allergic children from Australia, Ireland, and the United States.83
A more recent analysis assessed a dataset of over 2000 challenges to cow's milk from across the world (6% adults, 14% adolescents). There was little variation in ED01/ED05 with age, although a trend towards slightly lower values in 1–2 year olds was noted.84 The same dataset also allowed for an analysis of impact by geographical region (Japan, Australia, Europe, United States/Canada, Israel). While small differences were seen (most likely due to challenge protocol and cohort size in each region), the data provide reassurance that an ED05 of ∼2 mg cow's milk protein (as proposed by the FAO/WHO Expert Panel) would be protective across regions.84
Proposal to use 5 ppm as an action level
While VITAL® uses Action levels informed by doses (RfDs) as the basis for determining the need for PAL, others have proposed to use a concentration (similar to the approach adopted in Japanese legislation) which does not take into account the serving size a person might eat. Specifically, in 2022, some members of the Global Allergy and Asthma European Network (GA2LEN) proposed a cut-off of 5 ppm (0.5 mg protein/100 g food). This was on the basis of a review of the literature which identified no reports of these levels of exposure causing a life-threatening or fatal reaction.54 However, there was significant opposition to this.85 Given that food-allergic individuals react to actual amounts of allergen (rather than a concentration), the use of a concentration (independent of serving size) thus attracts the same criticisms as VITAL® 1.0. It is not possible to measure 5 ppm reliably with existing analytical tools86 — a key consideration for industry and regulators — and a cut-off of 0.5 mg/100 g is unduly restrictive for many allergens. Finally, the proposal did not address the needs of food-allergic consumers to have their risk of any allergic reaction minimised, and not “just” fatal reactions.
The debate is important in terms of 2 issues: (i) should a cut-off to inform PAL application be based on an actual exposure amount or a fixed concentration (such as 10 ppm), and (ii) is it better to have a single cut-off level for all allergens, or different cut-offs appropriate to individual allergens? Doses can be estimated (depending on the portion size, as per VITAL® program and the FAO/WHO Expert Consultation87) or accurately documented as total amounts of allergenic protein (something only done with formal OFC). Concentrations are typically expressed as mg allergenic protein per kilogram food (ie, parts per million (ppm)), and are used by analytical tools. Using a fixed concentration as a cut-off may be easier to apply in terms of monitoring UAP (within the limits of the technology used), but does not consider the serving size and thus the potential amount of exposure.
FAO/WHO expert consultation on risk assessment of food allergens, 2020–2023
In 2019, CCFL recognized the need to address inconsistencies in the use of PAL at a global level, and therefore requested FAO and WHO to provide scientific advice in this respect. The resulting Expert Consultation recommended an approach whereby.
-
•
The decision whether to use PAL (or not) should be based on a formal risk assessment, to assess for UAP despite adherence to allergen management practices and controls (see Fig. 13).
-
•
PAL should only be used in situations where UAP cannot be prevented, and could result in an exposure above the defined RfD for that allergen (see last column, Table 3)
-
•
The RfDs should primarily be informed by ED05 (the dose of allergenic protein that would be expected to elicit an objective allergic reaction in 5% of the population allergic to that allergen).
Fig. 13.
Recommended approach for PAL application in Codex.87
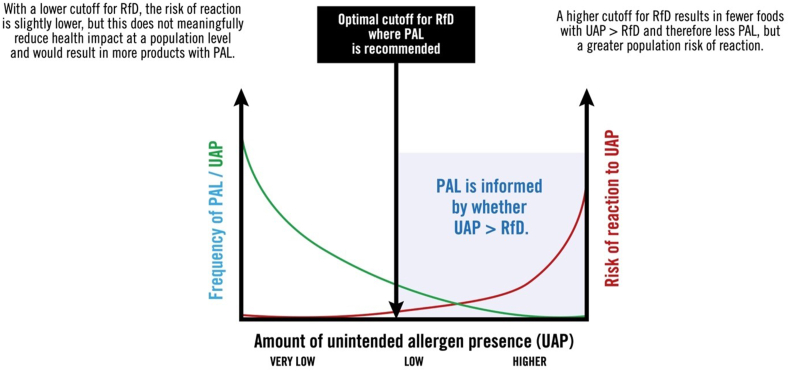
The decision to recommend RfDs informed by ED05 rather than ED01 was discussed at length. Setting a high Action Level would result in fewer products with UAP > RfD and therefore less PAL; however, this could result in products with no PAL but still with residual UAP that posed unacceptable health risks. Using a lower Action Level would result in more products with UAP > RfD, and therefore more PAL — and therefore would not necessarily lead to more meaningful PAL and/or a reduction in health impact at a population level (see Fig. 14).
Fig. 14.
Defining the optimal cut off for RfD to inform the most accurate PAL.
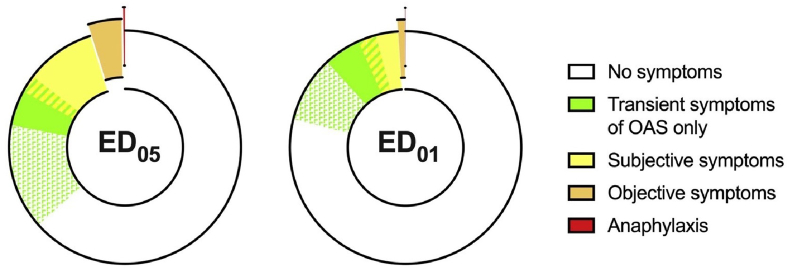
The Expert Group undertook an analysis of symptom severity due to low-level allergen exposure in people with IgE-mediated food allergy, where symptoms progress from very mild, transient symptoms such as oral pruritus to more significant subjective symptoms (eg, abdominal pain), objective signs (eg, urticaria, angioedema, vomiting) through to anaphylaxis. There were most data for peanut. While 5 times more people could react with objective symptoms to an ED05 compared to an ED01 exposure, this did not mean that 5 times more people would experience any symptoms; indeed, this proportion would only increase by around 50%, from 14 to 23% for ED01 to 20–35% for ED05 (Fig. 15). The Expert Group went on to review the rates of symptoms following ED05 exposures for other priority allergens, and concluded that the data supported the use of peanut as an exemplar allergen for this purpose.51
Fig. 15.
Frequency of allergic symptoms/signs following an ED05 and ED01 level of exposure in peanut-allergic individuals.79 While 5 times more people would have objective reaction to ED05 (compared to ED01), the rate of subjective symptoms is only increased by ∼50%.51
The Expert Group therefore recommended that ED05 should be used to inform RfDs,51 rather than a more conservative cut-off.
-
1.
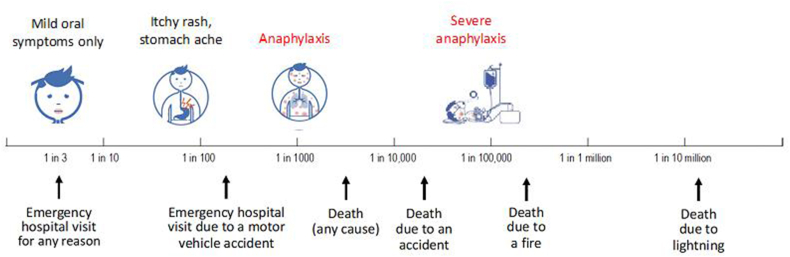
After considering the proportion of individuals potentially affected by reactions at ED01 and ED05 exposures, and their severity including the absence of reports of fatal or severe anaphylaxis (Fig. 16), the Expert Committee concluded that the stated safety objective (“to minimise, to a point where further refinement does not meaningfully reduce health impact, the probability of any clinically relevant objective allergic response following exposure to unintended presence of allergens”) would be met by using the ED05 as the basis for defining RfDs.
-
2.
There was more precision (and thus less uncertainty) over ED05 than for ED01 values.
-
3.
Current analytical capabilities can detect ED05-levels for most priority food allergens, (although other limitations remain), but not levels below ED05.86 Using ED05-based RfDs would facilitate laboratory validation, something that may be desired by regulators and industry to inform risk assessment. Indeed, many manufacturers use internal levels for implementation to ensure compliance – something feasible with ED05 but not with ED01.
-
4.
ED01 is less verifiable, and therefore more difficult to monitor. Food businesses may not feel confident in implementing a risk management plan based on ED01; the potential costs of food recalls makes it likely that they would err on the side of caution and apply PAL even when no real risk is posed. Using more conservative RfDs based on ED01 could therefore paradoxically increase PAL use.
Fig. 16.
Risks associated with an ED05 level of exposure in the general food-allergic population, relative to other health risks.
The FAO/WHO Expert Group also considered whether data derived from OFC are applicable to “real world” exposures. Administering incremental doses during OFC is different to ingestion of (typically a single dose) during real-world exposures, and could theoretically induce transient desensitisation and overestimate the reaction threshold.88 However, evidence suggests this does not occur in most individuals.89 Furthermore, ED01/05 levels of exposure tend to be equivalent to the first dose(s) during OFC, and therefore not impacted by dose titration. ED05 estimates for peanut and cow's milk have also been validated using single-dose challenges, which also provides much more certainty over current estimates.83,90 A second issue relates to how food processing, and inclusion of allergen in a complex food “matrix”, impacts allergenicity and allergen absorption. However, given that most challenge protocols use unprocessed allergen, this is not usually of concern because processing generally results in reduced allergenicity (a key exception being peanut, which is addressed by the use of roasted rather than raw peanut for OFC). Indeed, data suggests that incorporation of egg or milk into baked matrices has little impact on ED01/05 thresholds.91
Remaining obstacles to a regulatory approach for PAL
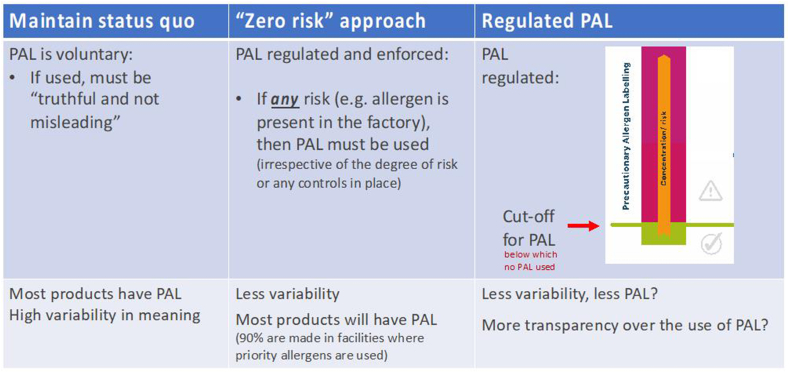
Arguably, there is now a global consensus that the status quo for PAL is no longer sustainable. Currently, application of PAL is widespread, inconsistent and causes confusion. A “zero-risk” approach might be initially attractive to allergic consumers, but in practice would result in even more products with PAL and therefore of little benefit (indeed, many would see this as a significant step backwards, particularly given that there has been a consensus for over a decade that “as with other risks in society, zero risk for food-allergic people is not a realistic or attainable option.”92 Therefore, the way forward has to be a regulated framework for application of PAL, which utilises a cut-off or reference dose below which PAL would not be required (Fig. 17).
Fig. 17.
Options to improve PAL.
The FAO/WHO Expert consultation has provided much “food for thought” in an evidence-based format, to underpin the global regulation of PAL. The reports generated by the consultation are currently being considered by CCFL as well as by industry stakeholders, and potential implementation will need buy-in from all stakeholders. Notwithstanding, at least 1 European Regulator (in the Netherlands) has already announced they will adopt the approach proposed by the FAO/WHO Expert group as an interim measure, with immediate effect.93 However, some outstanding issues need to be considered.
-
i)
Is a system based on ED05acceptable to all stakeholders, and what measures might be needed to help more sensitive allergic consumers who react to ED05exposures to avoid reactions?
ED05 levels of exposure will still result in objective symptoms in 5% of the allergic population, and up to one-third may experience (typically mild) subjective symptoms. These sub-populations can be identified through single-dose challenges,83,90 and diagnostic blood tests may soon be able to achieve the same result, at least for peanut.94 There are 3 key considerations.
-
•
Could individuals who might react to ED05-levels of exposure be readily identified in current healthcare systems, in a way that does not exacerbate existing disparities in healthcare due to socioeconomic factors?
-
•
How best to protect the most sensitive 5% of the allergic population who are at risk of objective symptoms of UAP ≤ ED05, as they would not know if a food product without PAL poses a risk to them or not.
-
•
When allergic individuals begin to experience mild symptoms following an allergen exposure, they do not know whether their only symptoms will be mild and transient oropharyngeal itch (likely with very small exposures) or whether their reaction may progress to anaphylaxis.
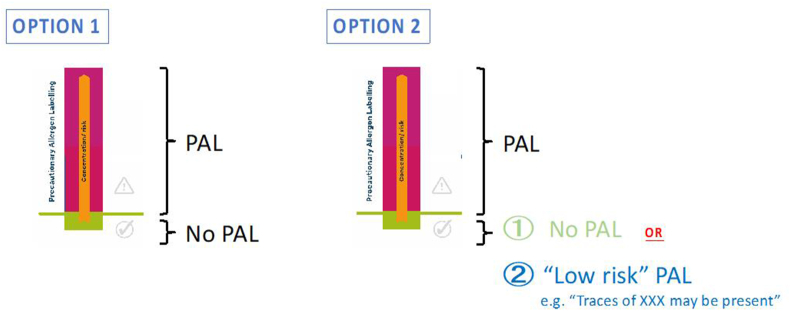
One solution discussed, but not supported, at the FAO/WHO Expert Consultation was to apply a specific statement to indicate UAP ≤ RfD (Fig. 18). The rationale is that consumers would still be alerted as to the potential of risk, but the wording would communicate that consumption is “safe” for 95% of allergic consumers. If a consumer knew that they did not normally react to ED05 levels of exposure but developed oral itch, they could be reassured that their reaction would be unlikely to progress. Ultimately, this option was not recommended, primarily because it would not lead to less use of PAL for negligible UAP which was a desired advantage for a risk-based PAL approach, ie. there is a risk that many food businesses might default to a “low risk” PAL. However, the Expert Group acknowledged that further work and stakeholder engagement is needed to understand what strategies would be acceptable to allergic consumers.
Fig. 18.
Two-level PAL which might provide protection to those allergic individuals who might experience symptoms to an exposure level ≤ ED05 (25–30% of allergic consumers would still have (mostly mild and transient) subjective symptoms, and 5% would have an objective allergic reaction).
At risk is the possibility that a small proportion of food products might have no PAL but cause (typically mild) objective reactions in 5% of the most sensitive allergic individuals, and mild subjective symptoms in up to 30% — as this could undermine consumer confidence in this approach. This risk – and potential solutions — needs to be considered by all stakeholders, including food businesses who may be concerned as to potential adverse impact on brand reputation were individuals to react to food products lacking PAL.
-
ii)
Should UAP only ever be communicated through PAL?
In some countries, UAP may be communicated through minor ingredients labelling or in certain countries, if UAP exceeds an action level (e.g. Japan). The inclusion of allergens in ingredients lists which may or may not be present due to UAP can cause confusion, reduces consumer trust in the value of ingredients lists, and may encourage risk-taking (if consumers ignore allergens listed as ingredients).
-
iii)
What wording or symbol should be applied for PAL?
The FAO/WHO Expert Group recommended the use of a single standardised statement which would convey that a product with PAL poses a health risk to individuals with an allergy to that particular food, and is thus not suitable for them. DunnGalvin et al. surveyed 1560 European adults with FA or parents of food-allergic children, and found that the most popular PAL phrasing was “not suitable for” (46%), closely followed by “may contain X” (44%).95 However, amongst members of the Expert Group, the wording “not suitable for” was not considered to accurately describe the risks from UAP present at the ED05 level but would be tolerated by (and thus “suitable” for) up to 95% of allergic consumers.87
A 2009 survey undertaken by the US FDA in 1243 individuals (of whom 739 made food purchases for food-allergic consumers) reported that while “may contain” was the most preferred wording, this choice was only expressed by one-third or respondents.96 A more recent US survey of food-allergic consumers reported that 29% preferred “Not suitable for …” while 22% preferred “May contain”.97 A recent survey from the United Kingdom also reported a preference for the simplest expression of PAL such as “May contain X”.98 Use of the term “traces” may lead consumers to believe that a food poses less of a risk “as it is only a trace”; likewise, people who experience symptoms to foods labelled “may contain traces” may believe they are more at risk of severe reactions as they reacted to a “trace".99 The Expert Group concluded that the term “trace” is usually ill-defined, and does not adequately convey that a risk assessment has found UAP which poses an appreciable risk. Therefore, the use of “traces” was not recommended. Whichever type of phrase or statement is chosen, it should effectively convey the message that the product may contain UAP at levels above appreciable risk, and should therefore not be consumed by allergic individuals. The precise wording of the single phrase for PAL should be decided by CCFL in conjunction with all relevant stakeholders and should consider local linguistic nuances.87
The Expert Group recommended that the application of PAL should be regulated.87 However, aware that this might not be adopted by CCFL, the Expert Group recommended that there should be some form of indication (such as a specific symbol) on packaging so that consumers would know that a formal risk assessment had been conducted to inform the need for PAL (something that would be unnecessary if all PAL were applied as part of a regulated framework).
-
iv)
What role would healthcare professionals have?
There is already a need for healthcare professionals to help allergic consumers how to interpret allergen labelling, including PAL. If a consistent and regulated approach were to be applied to PAL, then healthcare professionals would have a critical role in advising their patients, particularly those allergic individuals who might react to exposures below the RfD. Given the potential medicolegal concerns (which may be of greater relevance in some countries), there is a need for a consensus in terms of the advice these individuals should receive with respect to consuming food products with potential UAP ≤ RfD (and how to interpret a two-level PAL system (such as that proposed in Fig. 18), if such a scheme were implemented. Given the existing wide variation in advice given by healthcare professionals to patients,57,60 consistent education of allergic consumers and other stakeholders will be critical.
Whilst the majority of allergic consumers (and those choosing food on their behalf) desire more consistent and meaningful PAL, many are reluctant to purchase foods that might contain even a small amount of UAP. The potential for UAP to occur but not be declared on packaging may not be acceptable for many allergic consumers, partly because it is contradictory to previous public health communications that exposure to even a very small amount of allergens can be potentially fatal.
-
v)
Should the potential frequency of UAP be a factor in the application of PAL?
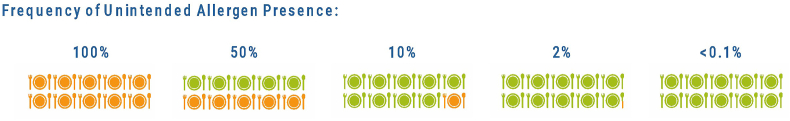
An important consideration is the frequency of the cross-contact problem that might lead to UAP. This will depend on the type of food being produced, and the equipment involved. UAP can vary from highly sporadic to frequent (Fig. 19). In contrast, the frequency of UAP due to particulates (e.g. whole seeds, chunks of nut) is much lower, and can be difficult to evaluate because it is usually non-uniform and occurs sporadically (Fig. 6). The current proposals being considered by CCFL assume that PAL should be applied whenever there is a risk of UAP > RfD; it does not take into consideration whether application of PAL should also depend on the frequency at which UAP might occur.
Fig. 19.
Is frequency of potential UAP an important consideration when determining the need for PAL?.
If application of PAL becomes regulated, then it will be necessary for regulators to include guidelines as to how to conduct the risk assessment (and the use or not of sampling plans to verify the assessment together with how to deal with analytical uncertainties). These guidelines will need to be developed for each industrial sector (e.g. bakery products, meat processing, etc) in close collaboration with food businesses. Justification of PAL use should be based on evidence that cross-contact does occur, and can exceed the action level (such evidence can include visual inspection, weighing of ingredients, analysis of allergens, etc). While the principles of the risk assessment should be the same across industry sectors, recommendations for implementation may need to be specific and therefore not straightforward. Given that zero risk is not possible, there is potential uncertainty over the legal consequences of a regulated system where UAP might cause an allergic reaction, but the regulated framework did not require PAL.
-
vi)
How might “free-from” claims fit into any regulated framework?
“Vegan” and “Free from” allergen labelling presents a different challenge to PAL since “free” is often taken to mean completely absent, or not detectable, rather than below a numerical threshold. However, in most regions, from the legal perspective, these are considered “marketing” statements. “Vegan” and “Free-from” labels exist in a legal grey-zone: while products labelled in this way should not contain an ingredient that contradicts the label (and thus comply with marketing claim), the “free from” or “vegan” claim does not extend to UAP. Thus, in many countries, "free-from" foods may also have a PAL to the allergen the product is “free-from”, Notably, in Germany, this scenario is prohibited, which might contribute to the relative paucity of “free-from” foods in this country. “Vegan” claims only indicate that no animal-derived ingredients are used in the food. It is for this reason that vegan or “free-from” products often have PAL, including allergens (like egg or milk) that are derived from animals. Consumers are generally not aware that “free-from” and “vegan” are considered marketing statements, and their use is not informed by food-safety legislation.
However, consumers often rely on vegan or “free-from” labels to guide allergen-free food choices. Vegan foods are often considered to be “safe” by those purchasing products where there is a need to avoid animal proteins (meat, poultry, fish/seafood, mammalian milk and eggs). The term "vegan" may be used as a shortcut for consumers who are less confident in explaining their food allergies to staff. A Canadian survey of consumers allergic to egg or cow's milk reported that 86% would consider “vegan” products to be “safe” for them.100 Surveys in both Canada and the United Kingdom have demonstrated that prepacked foods carrying vegan and/or “plant-based” labels can pose a significant risk, particularly to milk-allergic consumers.100,101 At least 1 fatality has been reported in the United Kingdom to a vegan wrap which contained “vegan” yoghurt with detectable cow's milk protein present.102
Further confusion can arise from the use of “gluten-free” claims: in many countries, these are regulated and subject to a maximum gluten presence of 20 ppm.103 Products containing 20 ppm or less can be labelled as gluten-free. However, a wheat-allergic person consuming a 200 g food serving which contains 20 ppm gluten (and can therefore be labelled as “gluten-free”) would consume around 5.5 mg total wheat protein – an exposure to which 2–6% of wheat-allergic individuals would be expected to have an objective allergic reaction, and 10–15% would be expected to experience mild subjective symptoms.76 Thus, “gluten-free” foods may not be appropriate for a proportion of wheat-allergic individuals who react to lower doses.
The fact that “gluten-free” claims have a regulated cut -off (and thus permit the presence of gluten≥20 ppm) should not be seen as setting a precedent for applying existing action levels to other allergens. At the inquest relating to the UK fatality due to “vegan” yoghurt in a wrap (referred to above), the manufacturer asserted that they could declare their product to be “milk-free” as it would not have had cow's milk protein present above an ED05 level, analogous to “gluten-free” labelling. However, the Coroner rejected this argument, clearly stating that “foods labelled in this way must be free from that allergen, and there should be a robust system to confirm the absence of the relevant allergen in all ingredients and during production when making such a claim.”102 The FAO/WHO Expert Consultation upheld the position stated by VITAL®, that Reference Doses are “not appropriate, nor intended, to be used to define ‘allergen-free’ labelling”.8
Conclusion
We have summarised developments in the application of PAL, including the recent FAO/WHO Expert Consultation, for healthcare professionals. CCFL are currently reviewing these recommendations. It is essential for healthcare professionals to provide input into this process, given their key role in supporting consumers with food allergy. We hope this review will provide a foundation for discussion and help build a consensus on how PAL can be better used to support safe food choices for allergic consumers.
Abbreviations
Act-up, World Allergy Organization Consensus on the Use of PAL; CCFL, Codex Committee on Food Labelling (CCFL); ED, Eliciting Dose; ED01, Eliciting Dose in 1% of patients; ED05, Eliciting Dose in 5% of patients; ED10, Eliciting Dose in 10% of patients; FA, food allergy; FALCPA, Food Allergen Labelling and Consumer Protection Act; FAO, Food and Agriculture Organization; FARRP, Food Allergy Research & Resource Program; FASTER, Food Allergy Safety, Treatment, Education, and Research Act; FDA, Food & Drug Administration; FDEIA, Food-Dependent Exercise-Induced Anaphylaxis; FDNIA, Food-Dependent Non-steroidal anti-inflammatory drug-Induced Anaphylaxis; GA2LEN, Global Allergy and Asthma European Network; GMP, Good Manufacturing Practices; GSLPF, General Standard for the Labelling of Prepackaged Foods; HACCP, Hazard Analysis Critical Control Point; LOAEL, Lowest Observed Adverse Effect Level; OFC, Oral Food Challenges; PAL, Precautionary Allergen Labelling (PAL); RA, Risk Assessment; RfD, Reference Dose; UAP, Unintended Allergen Presence; VITAL®, Voluntary Incidental Trace Allergen Labelling; VSEP, VITAL® Scientific Expert Panel; WAO, World Allergy Organization; WHO, World Health Organization.
Funding
ACT-UP! is a project of the World Allergy Organization.
Author contribution
AF, SS, JOBH, PE, and SLV drafted the first version of the manuscript. PT deeply revised and harmonized it. Each author contribute to revise all sections of the paper.
Ethics approval
Not applicable.
Consent for publication
All authors approved the final version and its submission.
Informed consent
Not applicable.
Declaration of competing interest
Paul J Turner: Grants from UK Medical Research Council, UK Food Standards Agency, JM Charitable Foundation, NIHR/Imperial Biomedical Research Centre and End Allergies Together, outside the submitted work; personal fees from UK Food Standards Agency, DBV Technologies, Aimmune Therapeutics, Allergenis and ILSI Europe outside the submitted work.
Antonio Bognanni: No conflicts to disclose.
Stefania Arasi: Advisory board member, consultant, and/or speaker for Novartis, DBV, Ferrero, and Ulrich outside the submitted work.
Ignacio J Ansotegui: Advisory board member, consultant, and/or speaker for Bayer, Bial, Cipla, Eurodrug, Faes Farma, Gebro, Glenmark, Menarini, MSD, Roxall and Sanofi outside the submitted work.
Sabine Schnadt: Speaker honoraria and advisory panel consultancy outside the submitted work for Aimmune and DBV.
Sébastien La Vieille: Employment: Health Canada (Government of Canada) - no conflict of interest.
Jonathan O’B Hourihane: Research funding from Johnson& Johnson, DBV Technologies; Travel support Stallergenes; Speaker fees Nutricia; Consultancy Camallergy, Stallergenes; Board membership Clemens von Pirquet Foundation and Irish Food Allergy Network.
Torsten Zuberbier: Institutional funding for research and/or honoria for lectures and/or consulting from Amgen, AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, FAES, HAL, Henkel, Kryolan, Leti, L'Oreal, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Stallergenes, Takeda, Teva and UCB, Uriach; in addition, he is a member of ARIA, DGAKI, ECARF, GA2LEN and WAO.
Philippe Eigenmann: Speaker and advisory board honoraria: DBV technologies, Novartis, ThermoFisher Scientific, Nestlé Health Sciences, Synlab, GSK,; Stocks and Stock options: DBV technologies.
Motohiro Ebisawa: No conflicts to disclose.
Mario Morais-Almeida: No conflicts to disclose.
Julie Barnett: Funded research from Food Standards Agency, UK. Member of Food Standards Agency Advisory Committee for Social Science.
Bryan Martin: No conflicts to disclose.
Linda Monaci: Speaker honoraria and funded research project by Ferrero outside the submitted work.
Graham Roberts: No conflicts to disclose.
Gary Wong: Advisory panel consultancy outside the submitted work for Haleon, Nestle, Novartis, OM Pharma, Ferrero.
Ruchi Gupta: Research support from the National Institutes of Health (NIH) (R21 ID # AI135705, R01 ID # AI130348, U01 ID # AI138907), Food Allergy Research & Education (FARE), Melchiorre Family Foundation, Sunshine Charitable Foundation, Novartis, and Genentech. Medical consultant/advisor for Genentech, Novartis, Food Allergy Research & Education (FARE), OWYN, Kaléo, Aquestive Therapeutics, and Byrn Pharma. Ownership interest in Yobee Care, Inc.
Sophia Tsabouri: No conflicts to disclose.
Clare Mills: No conflicts to disclose.
Simon Brooke-Taylor: Consults to and receives payment from the Allergen Bureau of Australia & New Zealand.
Consults on food regulation and compliance with Australian and New Zealand food standards to the food industry.
Joan Bartra: Speaker honoraria outside the submitted work for Thermo Fisher Scientific, Novartis, Menarini.
Michael Levin: Speaker honoraria and advisory panel consultancy outside the submitted work for Viatris, Novartis, Organon, Sanofi, Pfizer.
Marion Groetch: No commercial interests to disclose. Royalties from UpToDate and Academy of Nutrition and Dietetics and consulting fees from Food Allergy Research Education; serves on the Medical Advisory Board of IFPIES, as a Senior Advisor to FARE, as a Health Sciences Advisor for APFED.
Luciana Tanno: Speaker honoraria of Sanofi, DBV Technologies, Research grant from ANS (Agence Numerique de Santé), AllerGos.
Elham Hossny: No conflicts to disclose.
Barbara Ballmer Weber: Speaker honoraria and advisory panel consultancy outside the submitted work for Thermo Fisher Scientific, Novartis, ALK, Allergopharma, Menarini, Sanofi, MSD, Aiummune.
Vincenzo Fierro: Speaker honoraria for Stallergenes, Sanofi, GSK.
Benjamin C Remington: No conflicts to disclose.
Jennifer Gerdts: Food Allergy Canada receives unrestricted funding support for educational programming from Pfizer, DBV, Sanofi, American Peanut Council and consultant fees from Novartis.
M Hazel Gowland: Speaker honoraria: Food industry, technical and regulatory conferences, academic lecture fees Advisory panel consultancy: UK Food Standards Agency, Safefood, industry food technical and public health organisations.
Funded research: FSA, UKRI, Safefood funded studies, UK Fatal Anaphylaxis Registry.
Derek Chu: No conflicts to disclose.
Marjan Van Ravenhorst: Employee, Allergenen Consultancy BV.
Jennifer Koplin: No conflicts to disclose.
Alessandro Fiocchi: Speaker honoraria and advisory panel consultancy outside the submitted work for Nutricia, Abbott, Danone, Stallergenes, DBV, Novartis. Funded research (Institution) from Sanofi, Novartis, Ferrero, DBV, GSK, Astrazeneca, Hipp GmBDH, Humana SpA.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Allen K.J., Turner P.J., Pawankar R., et al. Precautionary labelling of foods for allergen content: are we ready for a global framework? World Allergy Organ J. 2014;7:10. doi: 10.1186/1939-4551-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DunnGalvin A., Chan C.H., Crevel R., et al. Precautionary allergen labelling: perspectives from key stakeholder groups. Allergy. 2015 Sep;70:1039–1051. doi: 10.1111/all.12614. [DOI] [PubMed] [Google Scholar]
- 3.Food and Agricultural Organization of the United Nations. Food safety and quality. Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens. Available at: https://www.fao.org/food-safety/scientific-advice/food-allergens/en/.
- 4.Prescott S.L., Pawankar R., Allen K.J., et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013 Dec 4;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren C.M., Jiang J., Gupta R.S. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. 2020;20:6. doi: 10.1007/s11882-020-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J., Ogorodova L.M., Mahesh P.A., et al. Comparative study of food allergies in children from China, India, and Russia: the EuroPrevall-INCO surveys. J Allergy Clin Immunol Pract. 2020;8:1349–1358. doi: 10.1016/j.jaip.2019.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R.S., Warren C.M., Smith B.M., et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2018.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner P.J., Baseggio Conrado A., Kallis C., et al. Time trends in the epidemiology of food allergy in England: an analysis of national data. Lancet Public Health. 2024;9:e664–e673. doi: 10.1016/S2468-2667(24)00163-4. [DOI] [PubMed] [Google Scholar]
- 9.Food Standards Agency . 2024. Patterns and prevalence of adult food allergy. [DOI] [Google Scholar]
- 10.Baseggio Conrado A., Patel N., Turner P.J. Global patterns in anaphylaxis due to specific foods: a systematic review. J Allergy Clin Immunol. 2021;148:1515–1525.e3. doi: 10.1016/j.jaci.2021.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta R.S., Springston E.E., Smith B., et al. Food allergy knowledge, attitudes, and beliefs of parents with food allergic children in the United States. Pediatr Allergy Immunol. 2010;21:927–934. doi: 10.1111/j.1399-3038.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- 12.DunnGalvin A., Dubois A.E., Flokstra-de Blok B.M., Hourihane J.O. The effects of food allergy on quality of life. Chem Immunol Allergy. 2015;101:235–252. doi: 10.1159/000375106. [DOI] [PubMed] [Google Scholar]
- 13.Warren C., Gupta R. Quality of life among food allergic patients and their caregivers. Curr Allergy Asthma Rep. 2016;16:38. doi: 10.1007/s11882-016-0614-9. [DOI] [PubMed] [Google Scholar]
- 14.Hu W., Grbich C., Kemp A. Parental food allergy information needs: a qualitative study. Arch Dis Child. 2007;92:771–775. doi: 10.1136/adc.2006.114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn-Galvin A. The impact of ‘labelling’ on the beliefs, attitudes and behaviours of consumers with food allergy: a multilevel perspective. Food Chem Funct Anal. 2020;22:127–140. [Google Scholar]
- 16.Codex Alimentarius general standard for labelling of pre-packaged foods (CODEX STAN 1-1985). 2010, pub WHO/FAO rome. Available at: ==%%%%%%%%%https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B1-1985%252FCXS_001e.pdf.
- 17.FAO and WHO Risk assessment of food allergens. Part 1 – review and validation of Codex Alimentarius priority allergen list through risk assessment. Meeting Report. Food Safety and Quality Series No. 14. Rome. 2022 doi: 10.4060/cb9070en. [DOI] [Google Scholar]
- 18.Commission Notice of 13 July 2017 Relating to the provision of information on substances or products causing allergies or intolerances as listed in Annex II to Regulation (EU) No 1169/2011 of the European Parliament and of the Council on the provision of food information to consumers. Off J Eur Union. 2017:5. [Google Scholar]
- 19.Food Sanitation Act (Act No. 233, 24 Feb 1947). Available at: https://www.cas.go.jp/jp/seisaku/hourei/data/fsa.pdf.
- 20.MFDS . Pub Ministry of Food and Drug Safety; Seoul: 2003. Korea Food & Drug Administration: Foods Labeling System.https://www.mfds.go.kr/eng/wpge/m_14/de011005l001.do [Google Scholar]
- 21.FALCPA. Food Allergen Labeling and Consumer Protection Act of 2004 (PublicLaw 108-282, Title II). 21 USC 301. Available at: https://public4.pagefreezer.com/browse/FDA/23-11-2021T07:28/https://www.fda.gov/food/allergens/food-allergen-labeling-and-consumer-protection-act-2004-falcpa, accessed May 6, 2024.
- 22.US Congress. S.578 - FASTER Act of 2021. Available at: https://www.congress.gov/bill/117th-congress/senate-bill/578.
- 23.US FDA. Guidance for Industry: Questions and Answers Regarding Food Allergen Labeling (Edition 5). Accessible at: https://www.fda.gov/food/guidance-documents-regulatory-information-topic/guidance-industry-questions-and-answers-regarding-food-allergens-including-food-allergen-labeling.
- 24.Food Allergen Labelling. Government of Canada; April 13, 2022. https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/allergen-labelling.html [Google Scholar]
- 25.FSANZ . Pub Commonwealth of Australia; 2022. Australia New Zealand Food Standards Code.http://www.foodstandards.gov.au/code/Pages/default.aspx [Google Scholar]
- 26.Turner P.J., Kemp A.S., Campbell D.E. Advisory food labels: consumers with allergies need more than “traces” of information. BMJ. 2011 Oct 13;343 doi: 10.1136/bmj.d6180. [DOI] [PubMed] [Google Scholar]
- 27.Madsen C.B., van den Dungen M.W., Cochrane S., et al. Can we define a level of protection for allergic consumers that everyone can accept? Regul Toxicol Pharmacol. 2020 Nov;117 doi: 10.1016/j.yrtph.2020.104751. [DOI] [PubMed] [Google Scholar]
- 28.Zurzolo G.A., Mathai M.L., Koplin J.J., Allen K.J. Precautionary allergen labelling following new labelling practice in Australia. J Paediatr Child Health. 2013 Apr;49:E306–E310. doi: 10.1111/jpc.12138. [DOI] [PubMed] [Google Scholar]
- 29.Manny E., La Vieille S., Barrere V., Théolier J., Godefroy S.B. Peanut and hazelnut occurrence as allergens in foodstuffs with precautionary allergen labeling in Canada. NPJ Sci Food. 2021 May 11;5:11. doi: 10.1038/s41538-021-00093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez S., Théolier J., Povolo B., Gerdts J., Godefroy S.B. Allergen management under a voluntary PAL regulatory framework — a survey of Canadian food processors. Heliyon. 2022 Nov 3;8 doi: 10.1016/j.heliyon.2022.e11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food Standards Agency Survey of allergen labelling and allergen content of processed foods. 2014. https://wwwhttps://www.food.gov.uk/research/food-allergy-and-intolerance-research/survey-of-allergen-labelling-and-allergen-content-of-processed-foods
- 32.Ontiveros N., Gallardo J.A., Arámburo-Gálvez J.G., et al. Characteristics of allergen labelling and precautionary allergen labelling in packaged food products available in Latin America. Nutrients. 2020 Sep 4;12:2698. doi: 10.3390/nu12092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pieretti M.M., Chung D., Pacenza R., Slotkin T., Sicherer S.H. Audit of manufactured products: use of allergen advisory labels and identification of labelling ambiguities. J Allergy Clin Immunol. 2009 Aug;124:337–341. doi: 10.1016/j.jaci.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Remington B.C., Baumert J.L., Marx D.B., Taylor S.L. Quantitative risk assessment of foods containing peanut advisory labelling. Food Chem Toxicol. 2013 Dec;62:179–187. doi: 10.1016/j.fct.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Aleccia J on behalf of Associated Press. New label law has unintended effect: Sesame in more foods. Published 21 Dec 2022. Available at: https://apnews.com/article/sesame-allergies-label-b28f8eb3dc846f2a19d87b03440848f1.
- 36.Food Standards Agency . 2007. Guidance on allergen management and consumer information.www.food.gov.uk/multimedia/pdfs/maycontainguide.pdf Available at: [Google Scholar]
- 37.Brough H.A., Turner P.J., Wright T., et al. Dietary management of peanut and tree nut allergy: what exactly should patients avoid? Clin Exp Allergy. 2015 May;45:859–871. doi: 10.1111/cea.12466. [DOI] [PubMed] [Google Scholar]
- 38.Crotty M.P., Taylor S.L. Risks associated with foods having advisory milk labeling. J Allergy Clin Immunol. 2010 Apr;125:935–937. doi: 10.1016/j.jaci.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Remington B.C., Baumert J., Blom M.W., et al. Zenodo. ILSI Europe Report Series, ILSI Europe. 2022. Practical guidance on the application of food allergen quantitative risk assessment. [DOI] [Google Scholar]
- 40.Food Standards Agency . 2014. Survey of allergen labelling and allergen content of processed foods.https://www.food.gov.uk/research/food-allergy-and-intolerance-research/survey-of-allergen-labelling-and-allergen-content-of-processed-foods Available at: [Google Scholar]
- 41.Food Safety Authority of Ireland (FSAI). Food Allergens & Labelling Survey 2011. Available at: https://www.fsai.ie/publications/food-allergens-and-labelling-survey.
- 42.Ford L.S., Taylor S.L., Pacenza R., Niemann L.M., Lambrecht D.M., Sicherer S.H. Food allergen advisory labeling and product contamination with egg, milk, and peanut. J Allergy Clin Immunol. 2010 Aug;126:384–385. doi: 10.1016/j.jaci.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 43.Hefle S.L., Furlong T.J., Niemann L., Lemon-Mule H., Sicherer S., Taylor S.L. Consumer attitudes and risks associated with packaged foods having advisory labeling regarding the presence of peanuts. J Allergy Clin Immunol. 2007 Jul;120:171–176. doi: 10.1016/j.jaci.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Pele M., Brohée M., Anklam E., Van Hengel A.J. Peanut and hazelnut traces in cookies and chocolates: relationship between analytical results and declaration of food allergens on product labels. Food Addit Contam. 2007 Dec;24:1334–1344. doi: 10.1080/02652030701458113. [DOI] [PubMed] [Google Scholar]
- 45.Remington B.C., Baumert J.L., Blom W.M., Houben G.F., Taylor S.L., Kruizinga A.G. Unintended allergens in precautionary labelled and unlabelled products pose significant risks to UK allergic consumers. Allergy. 2015 Jul;70:813–819. doi: 10.1111/all.12625. [DOI] [PubMed] [Google Scholar]
- 46.Sheth S.S., Wasserman S., Kagan R., et al. Role of food labels in accidental exposures in food-allergic individuals in Canada. Ann Allergy Asthma Immunol. 2010 Jan;104:60–65. doi: 10.1016/j.anai.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Zurzolo G.A., Allen K.J., Peters R.L., et al. Self-reported anaphylaxis to packaged foods in Australia. J Allergy Clin Immunol Pract. 2019 Feb;7:687–689. doi: 10.1016/j.jaip.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Graham F., Benhamou A.H., Liu Y.J., Caubet J.C., Eigenmann P.A. Real-life evaluation of tolerance to foods with precautionary allergen labelling in children with IgE-mediated food allergy. Allergy. 2023 Sep;78:2558–2561. doi: 10.1111/all.15821. Epub 2023 Jul 26. PMID: 37493219. [DOI] [PubMed] [Google Scholar]
- 49.Michelsen-Huisman A.D., van Os-Medendorp H., Blom W.M., et al. Accidental allergic reactions in food allergy: causes related to products and patient's management. Allergy. 2018 Dec;73:2377–2381. doi: 10.1111/all.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blom W.M., Michelsen-Huisman A.D., van Os-Medendorp H., et al. Accidental food allergy reactions: products and undeclared ingredients. J Allergy Clin Immunol. 2018 Sep;142:865–875. doi: 10.1016/j.jaci.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 51.FAO Risk Assessment of Food Allergens – Part 2: review and establish threshold levels in foods for the priority allergens. Meeting Report. Food Safety and Quality Series. 2022 doi: 10.4060/cc2946en. No. 15. Rome. [DOI] [Google Scholar]
- 52.Mortz C.G., Eller E., Garvik O.S., Kjaer H.F., Zuberbier T., Bindslev-Jensen C. Challenge-verified thresholds for allergens mandatory for labeling: how little is too much for the most sensitive patient? Allergy. 2024;79:1306–1316. doi: 10.1111/all.15870. [DOI] [PubMed] [Google Scholar]
- 53.Fiocchi A., Monaci L., De Angelis E., et al. Reactivity to allergenic food contaminants: a study on products on the market. Clin Transl Allergy. 2023 Sep;13 doi: 10.1002/clt2.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuberbier T., Dörr T., Aberer W., et al. Proposal of 0.5 mg of protein/100g of processed food as threshold for voluntary declaration of food allergen traces in processed food - a first step in an initiative to better inform patients and avoid fatal allergic reactions: a GA2LEN position paper. Allergy. 2022;77:1736–1750. doi: 10.1111/all.15167. [DOI] [PubMed] [Google Scholar]
- 55.Kemp S.F., Lockey R.F. Peanut anaphylaxis from food cross-contamination. JAMA. 1996 Jun 5;275(21):1636–1637. doi: 10.1001/jama.1996.03530450026022. [DOI] [PubMed] [Google Scholar]
- 56.Barnett J., Vasileiou K., Gowland M.H., Raats M.M., Lucas J.S. Beyond labelling: what strategies do nut allergic individuals employ to make food choices? A qualitative study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner P.J., Skypala I.J., Fox A.T. Advice provided by health professionals regarding precautionary allergen labelling. Pediatr Allergy Immunol. 2014 May;25:290–292. doi: 10.1111/pai.12178. [DOI] [PubMed] [Google Scholar]
- 58.Schaible A., Kabourek J., Elverson W., Venter C., Cox A., Groetch M. Precautionary allergen labeling: avoidance for all? Curr Allergy Asthma Rep. 2024 Mar;24:81–94. doi: 10.1007/s11882-024-01129-x. [DOI] [PubMed] [Google Scholar]
- 59.Marchisotto M.J., Harada L., Kamdar O., et al. Food allergen labeling and purchasing habits in the United States and Canada. J Allergy Clin Immunol Pract. 2017 Mar-Apr;5:345–351.e2. doi: 10.1016/j.jaip.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 60.Turner P.J., Allen K.J., Mehr S., Campbell D.E. Knowledge, practice, and views on precautionary allergen labeling for the management of patients with IgE-mediated food allergy—a survey of Australasian and UK health care professionals. J Allergy Clin Immunol Pract. 2016 Jan-Feb;4:165. doi: 10.1016/j.jaip.2015.09.003. 7.e14. [DOI] [PubMed] [Google Scholar]
- 61.La Vieille S., Hourihane J.O., Baumert J.L. Precautionary allergen labeling: what advice is available for health care professionals, allergists, and allergic consumers? J Allergy Clin Immunol Pract. 2023 Apr;11:977–985. doi: 10.1016/j.jaip.2022.12.042. [DOI] [PubMed] [Google Scholar]
- 62.Health Canada . March 2012. The use of food allergen precautionary statements on prepackaged foods.https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/label-etiquet/allergen/precaution_label-etiquette-eng.pdf Available at: [Google Scholar]
- 63.UK Food Standards Agency. Precautionary allergen labelling. Available at: https://www.food.gov.uk/business-guidance/precautionary-allergen-labelling.
- 64.Madsen C.B., Hattersley S., Buck J., et al. Approaches to risk assessment in food allergy: report from a workshop ‘‘developing a framework for assessing the risk from allergenic foods.”. Food Chem Toxicol. 2009;47:480–489. doi: 10.1016/j.fct.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Shoji M., Adachi R., Akiyama H. Japanese food allergen labeling regulation: an update. J AOAC Int. 2018 Jan 1;101:8–13. doi: 10.5740/jaoacint.17-0389. [DOI] [PubMed] [Google Scholar]
- 66.Akiyama H., Imai T., Ebisawa M. Japan food allergen labeling regulation--history and evaluation. Adv Food Nutr Res. 2011;62:139–171. doi: 10.1016/B978-0-12-385989-1.00004-1. [DOI] [PubMed] [Google Scholar]
- 67.Federal Department Affairs . 2005. Ordinance of the DFI on the labeling and advertising of foodstuffs (Ordinance 817.022.21)https://www.fedlex.admin.ch/eli/cc/2005/805/fr Available at: [Google Scholar]
- 68.Ballmer-Weber B.K. Allergic reactions to food proteins. Int J Vitam Nutr Res. 2011 Mar;81(2-3):173–180. doi: 10.1024/0300-9831/a000055. [DOI] [PubMed] [Google Scholar]
- 69.Czech Ministry of Agriculture. Providing information on the possible and intentional occurrence of substances or products causing allergies or intolerance in food. Available at: https://eagri.cz/public/portal/mze/potraviny/publikace-a-dokumenty/narodni-doporuceni-pro-uvadeni-informaci.html.
- 70.Brooke-Taylor S., Christensen G., Grinter K., Sherlock R., Warren L. The allergen Bureau VITAL program. J AOAC Int. 2018;101:77–82. doi: 10.5740/jaoacint.17-0392. [DOI] [PubMed] [Google Scholar]
- 71.Taylor S.L., Hefle S.L., Bindslev-Jensen C., et al. Factors affecting the determination of threshold doses for allergenic foods: how much is too much? J Allergy Clin Immunol. 2002;109:24–30. doi: 10.1067/mai.2002.120564. [DOI] [PubMed] [Google Scholar]
- 72.Buchanan R., Dennis S., Gendel S., et al. Threshold Working Group. Approaches to establish thresholds for major food allergens and for gluten in food. J Food Protect. 2008;71:1043–1088. doi: 10.4315/0362-028x-71.5.1043. [DOI] [PubMed] [Google Scholar]
- 73.Taylor S.L., Baumert J.L., Kruizinga A.G., et al. Establishment of reference doses for residues of allergenic foods: report of the VITAL Expert Panel. Food Chem Toxicol. 2014;63:9–17. doi: 10.1016/j.fct.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 74.Crevel R.W., Ballmer-Weber B.K., Holzhauser T., et al. Thresholds for food allergens and their value to different stakeholders. Allergy. 2008 May;63:597–609. doi: 10.1111/j.1398-9995.2008.01636.x. [DOI] [PubMed] [Google Scholar]
- 75.Wheeler M.W., Westerhout J., Baumert J.L., Remington B.C. Bayesian stacked parametric survival with frailty components and interval-censored failure times: an application to food allergy risk. Risk Anal. 2021 Jan;41:56–66. doi: 10.1111/risa.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houben G.F., Baumert J.L., Blom W.M., et al. Full range of population Eliciting Dose values for 14 priority allergenic foods and recommendations for use in risk characterization. Food Chem Toxicol. 2020 Dec;146 doi: 10.1016/j.fct.2020.111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel N., Adelman D.C., Anagnostou K., et al. Using data from food challenges to inform management of consumers with food allergy: a systematic review with individual participant data meta-analysis. J Allergy Clin Immunol. 2021 Jun;147(6):2249–2262.e7. doi: 10.1016/j.jaci.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner P.J., Patel N., Campbell D.E., et al. Reproducibility of food challenge to cow's milk: systematic review with individual participant data meta-analysis. J Allergy Clin Immunol. 2022 Nov;150(5):1135–1143.e8. doi: 10.1016/j.jaci.2022.04.035. [DOI] [PubMed] [Google Scholar]
- 79.Dua S., Ruiz-Garcia M., Bond S., et al. Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: a randomized controlled study. J Allergy Clin Immunol. 2019 Dec;144(6):1584–1594.e2. doi: 10.1016/j.jaci.2019.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner P.J., Patel N., Ballmer-Weber B.K., et al. Peanut can Be used as a reference allergen for hazard characterization in food allergen risk management: a rapid evidence assessment and meta-analysis. J Allergy Clin Immunol Pract. 2022 Jan;10:59–70. doi: 10.1016/j.jaip.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bartra J., Turner P.J., Muñoz-Cano R.M. Cofactors in food anaphylaxis in adults. Ann Allergy Asthma Immunol. 2023 Jun;130(6):733–740. doi: 10.1016/j.anai.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 82.Allen K.J., Remington B.C., Baumert J.L., et al. Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications. J Allergy Clin Immunol. 2014 Jan;133(1):156–164. doi: 10.1016/j.jaci.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 83.Hourihane J.O., Allen K.J., Shreffler W.G., et al. Peanut Allergen Threshold Study (PATS): novel single-dose oral food challenge study to validate eliciting doses in children with peanut allergy. J Allergy Clin Immunol. 2017 May;139:1583–1590. doi: 10.1016/j.jaci.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 84.Turner P.J., Bindslev-Jensen C., Campbell D.E., et al. Impact of age on eliciting dose to cow’s milk at food challenge: analysis of a global dataset. Presented at the World Allergy Congress. 2024 [Google Scholar]
- 85.Turner P.J., Baumert J.L., Beyer K., et al. EAACI Taskforce on Food allergen thresholds. 'Too high, too low': the complexities of using thresholds in isolation to inform precautionary allergen ('may contain') labels. Allergy. 2022;77:1661–1666. doi: 10.1111/all.15202. [DOI] [PubMed] [Google Scholar]
- 86.Holzhauser T., Johnson P., Hindley J.P., et al. Are current analytical methods suitable to verify VITAL® 2.0/3.0 allergen reference doses for EU allergens in foods? Food Chem Toxicol. 2020 Nov;145 doi: 10.1016/j.fct.2020.111709. [DOI] [PubMed] [Google Scholar]
- 87.FAO & WHO . 2023. Risk assessment of food allergens – Part 3: review and establish precautionary labelling in foods of the priority allergens, Meeting report. Food Safety and Quality Series No. 16. Rome. [DOI] [Google Scholar]
- 88.Niggemann B., Lange L., Finger A., Ziegert M., Müller V., Beyer K. Accurate oral food challenge requires a cumulative dose on a subsequent day. J Allergy Clin Immunol. 2012 Jul;130:261–263. doi: 10.1016/j.jaci.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 89.Álvarez García O., Bartra J., Ruiz-Garcia M., et al. No apparent impact of incremental dosing on eliciting dose at double-blind, placebo-controlled peanut challenge. Allergy. 2022 Feb;77:667–670. doi: 10.1111/all.15130. [DOI] [PubMed] [Google Scholar]
- 90.Turner P.J., d'Art Y.M., Duca B., et al. Single-dose oral challenges to validate eliciting doses in children with cow's milk allergy. Pediatr Allergy Immunol. 2021 Jul;32:1056–1065. doi: 10.1111/pai.13482. [DOI] [PubMed] [Google Scholar]
- 91.Remington B.C., Westerhout J., Campbell D.E., Turner P.J. Minimal impact of extensive heating of hen's egg and cow's milk in a food matrix on threshold dose-distribution curves. Allergy. 2017 Nov;72:1816–1819. doi: 10.1111/all.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madsen C.B., Hattersley S., Allen K.J., et al. Can we define a tolerable level of risk in food allergy? Report from a EuroPrevall/UK Food Standards Agency workshop. Clin Exp Allergy. 2012 Jan;42:30–37. doi: 10.1111/j.1365-2222.2011.03868.x. [DOI] [PubMed] [Google Scholar]
- 93.Fnli C.B.L., NVWA Guidelines on cross-contact of allergens. 2024. https://fnli.nl/actueel/richtlijnendocument-geeft-bedrijven-handvatten-voor-het-nieuwe-allergenenbeleid
- 94.Suprun M., Kearney P., Hayward C., et al. Predicting probability of tolerating discrete amounts of peanut protein in allergic children using epitope-specific IgE antibody profiling. Allergy. 2022 Oct;77:3061–3069. doi: 10.1111/all.15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DunnGalvin A., Roberts G., Regent L., et al. Understanding how consumers with food allergies make decisions based on precautionary labelling. Clin Exp Allergy. 2019 Nov;49(11):1446–1454. doi: 10.1111/cea.13479. [DOI] [PubMed] [Google Scholar]
- 96.Verrill L., Choinière C.J. Are food allergen advisory statements really warnings? Variation in consumer preferences and consumption decisions. J Food Prod Market. 2009;15(2):139–151. doi: 10.1080/10454440802316800. [DOI] [Google Scholar]
- 97.Gupta R., Kanaley M., Negris O., Roach A., Bilaver L. Understanding precautionary allergen labeling (PAL) preferences among food allergy stakeholders. J Allergy Clin Immunol Pract. 2021;9:254–264. doi: 10.1016/j.jaip.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 98.UK Food Standards Agency. Consultation on Food Allergen Labelling and Information Requirements Technical Guidance: Summary of stakeholder responses. https://www.food.gov.uk/business-guidance/consultation-on-food-allergen-labelling-and-information-requirements-technical-guidance-summary-of-stakeholder-responses.
- 99.Turner P.J., Gowland M.H. Precautionary allergen labelling: no more traces. Allergy. 2016 Oct;71:1505–1507. doi: 10.1111/all.12961. [DOI] [PubMed] [Google Scholar]
- 100.Dominguez S., Théolier J., Lizée K., Povolo B., Gerdts J., Godefroy S.B. "Vegan" and "plant-based" claims: risk implications for milk- and egg-allergic consumers in Canada. Allergy Asthma Clin Immunol. 2023 Aug 24;19:74. doi: 10.1186/s13223-023-00836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chartered Trading Standards Institute . 2023. Vegan and plant-based food.https://www.tradingstandards.uk/media/3179000/ctsi-vegan-plant-based-food-policy-paper-final.pdf Available at: [Google Scholar]
- 102.Voison M. 21 November 2022. Celia marsh: prevention of future deaths (regulation 28) report.https://www.judiciary.uk/wp-content/uploads/2022/11/Celia-Marsh-Prevention-of-future-deaths-report-2022-0379_Published.pdf [Google Scholar]
- 103.US Food & Drug Administration . June 2018. Gluten and food labeling.https://www.fda.gov/media/91945/download Available at: [Google Scholar]