Abstract
OmpU porins are increasingly recognized as key determinants of pathogenic host Vibrio interactions. Although mechanisms remain incompletely understood, various species, including the human pathogen Vibrio cholera, require OmpU for host colonization and virulence. We have shown previously that OmpU is essential for virulence in the oyster pathogen Vibrio splendidus LGP32. Here, we showed that V. splendidus LGP32 invades the oyster immune cells, the hemocytes, through subversion of host-cell actin cytoskeleton. In this process, OmpU serves as an adhesin/invasin required for β-integrin recognition and host cell invasion. Furthermore, the major protein of oyster plasma, the extracellular superoxide dismutase Cg-EcSOD, is used as an opsonin mediating the OmpU-promoted phagocytosis through its RGD sequence. Finally, the endocytosed bacteria were found to survive intracellularly, evading the host defense by preventing acidic vacuole formation and limiting reactive oxygen species production. We conclude that (i) V. splendidus is a facultative intracellular pathogen that manipulates host defense mechanisms to enter and survive in host immune cells, and (ii) that OmpU is a major determinant of host cell invasion in Vibrio species, used by V. splendidus LGP32 to attach and invade oyster hemocytes through opsonisation by the oyster plasma Cg-EcSOD.
Keywords: host–pathogen interaction, innate immunity, invertebrate, mollusk, oxidative burst
The oyster pathogen, Vibrio splendidus strain LGP32 was isolated from massive mortality events in the production of Crassostrea gigas oysters (1). However, up to now, little has been known about the route of infection and pathogenic processes of LGP32 (2, 3). A metalloprotease has been associated with toxicity (4, 5) and the outer membrane protein (OMP) OmpU was shown to be a major determinant of LGP32 virulence (6).
As bacterial surface components, OMPs are both used by hosts for pathogen recognition and by pathogens for interaction with and invasion of host cells, serving as adhesion proteins (adhesins) (7–9) or invasion proteins (invasins) (10, 11). OmpU is a major porin from the Vibrio species. Recent studies have shown that it mediates host–Vibrio interactions, being involved in the resistance to antimicrobial peptides (AMPs) (12, 13), the adherence to host cells (14), or pathogen/symbiont recognition (15). Similarly, in LGP32, OmpU participates to the resistance to oyster AMPs and displays adhesive properties (6). To date, little is known on the molecular basis of the OmpU-mediated host–Vibrio interactions. However, in Vibrio vulnificus, OmpU was reported to bind to fibronectin as a process for invasion of HEp-2 cells (14).
Hemocytes are the immune cells of mollusks. As such, they are equipped with immune receptors and effectors involved in pathogen recognition and control. Recent advances in mollusk–pathogen interactions have shown the existence of highly diverse somatically generated receptors that give high specificity to pathogen recognition (16, 17). So far, in oysters, recognition is mediated by germ-line encoded receptors, such as a β-integrin, involved in hemocyte phagocytosis (18); it was proposed to serve as a receptor for the major plasma protein, the extracellular superoxide dismutase Cg-EcSOD, which contains a RGD cell-binding domain (19). In addition, a broad diversity of immune effectors has been characterized in oyster hemocytes ranging from reactive oxygen species (ROS) (20, 21) to AMPs (22–24).
Some oyster pathogens target hemocytes to invade their host. For example, the parasite Perkinsus marinus enters C. virginica hemocytes through recognition by a specific receptor, the Cv-Gal galectin, which promotes its phagocytosis (25). Inside hemocytes, P. marinus avoids the induction of the oxidative burst as a mechanism of immune evasion, proliferates, and spreads throughout the host (26, 27).
Here, we have studied the role of OmpU in the interaction of LGP32 with oyster hemocytes. GFP-expressing derivatives of LGP32 and its ΔompU mutant were constructed. Through in vitro hemocyte invasion assays and experimental infections, we showed that LGP32 is a facultative intracellular pathogen that invades the oyster immune cells by using OmpU as an adhesin and invasin. In this process, the plasma Cg-EcSOD is used as an opsonin recognized through its RGD sequence by hemocyte β-integrins. OmpU-recognition was shown to subvert the host-cell actin cytoskeleton, inducing the expression of host-cell trafficking genes and resulting in actin and clathrin polymerization. Capable of intracellular survival, LGP32 was shown to escape from host cellular defenses by avoiding acidic vacuole formation and by limiting ROS production.
Results
V. splendidus LGP32 Invades and Survives in Oyster Hemocytes, Impairing Immune Defense Functions.
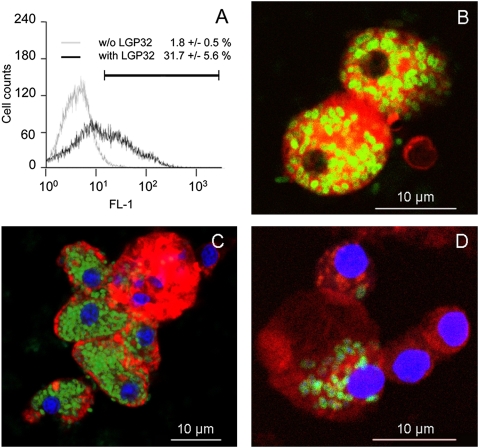
A GFP-expressing LGP32 was constructed that carries the gfp3 gene under the PTRC constitutive promoter in a genomic locus. After a 2-h in vitro contact between the GFP-expressing LGP32 and oyster hemolymph, 31.7 ± 5.6% hemocytes were GFP+, as determined by flow cytometry (Fig. 1A). A massive engulfment of LGP32 was observed on confocal microscopy optical sections (Fig. 1 B and C). Although less intense, the engulfment of GFP-expressing LGP32 by hemocytes was also observed in vivo 1 h after injection to oysters (Fig. 1D). Interestingly, counts of intracellular Vibrios monitored by plating hemocyte lysates from in vitro contacts were stable at 106 cfu/mL over 16 h (Fig. 2A). This finding showed that LGP32 remains viable and culturable within oyster hemocytes. In contrast, the avirulent control LMG20012T (6, 28), which was also avidly engulfed, did not survive within hemocytes, with cfu counts dropping by 4 logs over time (Fig. 2A). The bacterial load related to the oyster-associated environmental microflora remained below 10 cfu/mL over the time course.
Fig. 1.
LGP32 invades oyster hemocytes. (A) Flow cytometry analysis of hemocyte invasion by GFP-expressing LGP32. Cytograms show hemocyte cell counts vs. GFP-fluorescence (FL-1) after contact with LGP32 (black curve) or not (gray curve). The percentage of GFP+ hemocytes ± SEM is indicated. Data are representative of three independent experiments. (B–D) Confocal microscopy optical sections of invaded hemocytes. (B) Evan's blue-stained hemocytes (red) invaded by GFP-expressing LGP32 (green). (C and D) GFP-expressing LGP32 (green) in hemocytes upon phalloidin staining of actin (red) and DAPI staining of nucleic acids (blue). A, B, and C show in vitro data; D shows in vivo data.
Fig. 2.
LGP32 survives in oyster hemocytes. (A) Culturable intracellular bacteria (cfu/mL) in hemocytes. LGP32 (black), LMG20012T (gray), and control without bacteria (white). (B) Acidic vacuole formation (Lysotracker) and ROS production (DCFH-DA) in hemocytes after a 1-h contact with LMG20012T (gray) or LGP32 (black). Results are expressed in a relative fluorescent arbitrary unit (AU) after subtracting the fluorescence from the control without bacteria. All data are representative of three independent experiments (*P < 0.05).
Hemocyte responses were measured 1 h after engulfment of LGP32 or LMG20012T, when similar intracellular bacterial loads (105 to 106 cfu/mL) are present in both samples (Fig. 2A). Unchallenged hemocytes were used as a control. Interestingly, the lysotracker fluorescence indicative of acidic vacuole formation was similar in control and LGP32-containing hemocytes but significantly higher in LMG20012T-containing hemocytes (P < 0.05) (Fig. 2B). Similarly, the 2',7'-dichlorfluorescein-diacetate (DCFH-DA) fluorescence indicative of ROS production was significantly higher in LMG20012T- than in LGP32-containing hemocytes (P < 0.05), although both were higher than in control hemocytes (Fig. 2B). Thus, defense reactions involved in the efficient control of phagocytosed bacteria, such as LMG20012T, are significantly attenuated in hemocytes invaded by LPG32.
LGP32 Uses β-Integrin Recognition and Subverts the Host-Cell Actin Cytoskeleton for Hemocyte Invasion.
The role of β-integrin in recognition and binding of LGP32 was studied by incubating oyster hemolymph with anti–β-integrin or an irrelevant antibody before contact with GFP-expressing LGP32. Strikingly, flow cytometry counts of GFP+ hemocytes dropped by 72% upon anti–β-integrin treatment (P < 0.001), whereas they remained stable with the irrelevant antibody (Fig. 3A). Similarly, incubation of hemolymph with an Arg-Gly-Asp (RGD) tripeptide inhibited invasion by 72% (P < 0.01), but an irrelevant Glu-Gly-Phe (EGF) tripeptide had no significant effect (Fig. 3B). This result showed that the RGD-recognition domain of β-integrin is essential for hemocyte invasion.
Fig. 3.
Integrin- and RGD-dependent hemocyte invasion by LGP32 uses the polymerization of clathrin and actin microfilaments. Histograms display the inhibition of hemocyte invasion (%) as measured by flow cytometry after treatment of hemolymph with (A) anti–β-integrin or an irrelevant antibody (8 μg/mL), (B) an RGD or an EGF tripeptide (0.4 mg/mL), (C) sucrose (0.1, 0.2, and 0.5 M), and (D) cytochalasin D (1, 5, and 10 μg/mL). The 100% reference was attributed to untreated controls. Data are representative of three independent experiments. Statistical differences with untreated controls are displayed (*P < 0.05, **P < 0.01, and *** P < 0.001).
Contacts between oyster hemolymph and GFP-expressing LGP32 were then performed in the presence of hypertonic concentrations of sucrose, which inhibit receptor-mediated phagocytosis by inducing abnormal clathrin polymerization (29). Sucrose inhibited hemocyte invasion in a dose-dependent manner, from 24% (P < 0.01) at 0.1 M up to 68% (P < 0.001) at 0.5 M (Fig. 3C). Similarly, cytochalasin D used as an inhibitor of actin polymerization inhibited hemocyte invasion from 13% at 1 μg/mL (P < 0.05) to 71% at 10 μg/mL (P < 0.001) (Fig. 3D). This finding showed that hemocyte invasion by LGP32 uses host cell clathrin and actin polymerization.
Hemocyte Invasion Requires the OmpU Porin and a Plasma Opsonin That Specifically Binds OmpU.
The ability of an avirulent ΔompU mutant (6) to invade hemocytes was studied by constructing a GFP-expressing derivative. Hemocyte–Vibrio interactions were monitored by flow cytometry. Strikingly, the number of GFP+ hemocytes shifted from 31.7 ± 5.6% when exposed to LGP32 and down to 2.5 ± 1% when exposed to the ΔompU mutant (P < 0.01) (Fig. 4A). In agreement, confocal microscopy showed little to no intracellular GFP-expressing bacteria (Fig. 4B), indicating that OmpU is required for hemocyte invasion. Furthermore, invasion of hemocytes by wild-type LGP32 dropped dramatically from 31.7 ± 5.6% to 7.5 ± 2.3% (P < 0.05) upon plasma withdrawal, and returned to 32.0 ± 6.0% when plasma was added back (Fig. 4A and Fig. S1). This finding strongly suggests that a plasma component operates as an opsonin. To determine how OmpU and plasma are involved in the host–pathogen interaction, we tested the ability of LGP32 and ΔompU to bind to immobilized plasma proteins. Results showed that OmpU is required for the dose-dependent binding of LGP32 to plasma (Fig. S2). Taken together, these data strongly suggest that a plasma opsonin mediates recognition and promotes phagocytosis of LGP32 by binding to OmpU.
Fig. 4.
Hemocyte invasion by LGP32 is OmpU- and plasma-dependent. (A) Percentage of GFP+ hemocytes (flow cytometry data) obtained by incubating hemolymph, washed hemocytes, or washed hemocytes supplemented with plasma or purified Cg-EcSOD, with wild-type (black bars) or ΔompU (white bars) GFP-expressing LGP32. Data are representative of three independent experiments (*P < 0.05, **P < 0.01). (B) Confocal microscopy optical sections of Evan's blue counterstained hemocytes after exposure to wild-type or ΔompU GFP-expressing LGP32.
Oyster Cg-EcSOD Binds to OmpU in an RGD-Dependent Manner and Promotes Hemocyte Invasion by LGP32.
To identify OmpU-binding plasma proteins, plasma was fractionated by HPLC and fractions were tested for binding to GFP-expressing LGP32 and ΔompU. Only three HPLC fractions, referred to as f6, f7, and f8, showed differential binding (P < 0.05) (Fig. 5 A and C). On SDS/PAGE, they displayed a 30 kDa band predominant in f6 and f7, which was absent from the fractions displaying no differential binding (Fig. 5B). An LC-MS/MS analysis of f6 and f7 unambiguously identified the proteins as variants of Cg-EcSOD (GenBank AAY60161.1) (Fig. S3). The aberrant migration of Cg-EcSOD variants at ∼30 kDa instead of 20 kDa is in agreement with previous studies (19, 30). Fractions f6 and f7, which could contain different variants or foldamers of Cg-EcSOD, as presumed from their different chromatographic behavior (31), displayed similar binding to LGP32 (Fig. 5C). The binding of LGP32 to immobilized Cg-EcSOD was OmpU-dependent, dose-dependent, and linear in the range of concentrations tested (Fig. 6). Preincubation of LGP32 with RGD tripeptide resulted in a significant loss of binding by more than twofold (P < 0.01), but an EGF tripeptide (control) had no significant effect (Fig. 6). Taken together, these data show that the RGD sequence of Cg-EcSOD is essential for the OmpU-mediated binding of Cg-EcSOD to LGP32.
Fig. 5.
Identification of the OmpU-binding proteins. (A) Plasma proteins separated by RP-HPLC on a linear gradient of 0 to 70% ACN in 35 min. Fractions are referred to as f1 to f9. Sequences of trypsic fragments from OmpU-binding fractions f6 and f7 are shown. (B) Coomassie blue-stained SDS/PAGE (12%) of HPLC fractions. Proteins found in OmpU-binding fractions only are in a box. (C) Binding of GFP-expressing wild-type and ΔompU LGP32 to the immobilized HPLC fractions. Black bars refer to wild-type LGP32 and white bars to ΔompU. Results are expressed in a relative fluorescent arbitrary unit after subtracting the fluorescence from the control without bacteria. Significant difference in binding to wild-type and ΔompU is indicated (*P < 0.05, **P < 0.01).
Fig. 6.
OmpU-dependent binding of LGP32 to immobilized Cg-EcSOD requires the RGD sequence. The relative fluorescence caused by binding is compared for GFP-expressing LGP32 (black), RGD-treated (gray), and EGF-treated (hatches) LGP32, and untreated ΔompU (white). Data are representative of three independent experiments (*P < 0.05, **P < 0.01).
This finding prompted us to investigate the role of Cg-EcSOD in the plasma opsonisation previously observed. GFP-expressing wild-type or ΔompU LGP32 were treated with purified Cg-EcSOD before being exposed to washed hemocytes, unable to be invaded by LGP32. This process fully restored the ability of LGP32 to invade oyster hemocytes, as evidenced by both flow cytometry (Fig. 4A) and confocal microscopy (Fig. S1). Taken together, these results identify Cg-EcSOD as an oyster opsonin recognizing OmpU of LGP32.
Expression of Cell Remodeling Genes Is OmpU-Dependent in LGP32 Experimentally Infected Oysters.
To determine the role of OmpU on hemocyte responses in vivo, oysters were infected with 108 cfu of wild-type or ΔompU LGP32, or sterile sea water (SSW) (control). Expression of oyster hemocyte genes involved in actin dynamics and cell motility was monitored 24 h postinjection. Among them, β-actin is involved in cell motility, structure, and integrity through microfilament building. Intersectin-1 is a cytoplasmic membrane-associated protein that coordinates endocytic membrane traffic with the actin assembly machinery, and regulates the formation of clathrin-coated vesicles (32). Fascin is an actin cross-linking protein involved in the assembly of actin filaments into bundles (33). Finally, calreticulin is a multifunctional protein that serves as a cell-surface receptor for the C1q component of complement and mediates phagocytosis upon binding to major plasma proteins (34, 35). Although the number of actin transcripts was similar in Vibrio- and SSW-injected oysters, all other three genes had a significantly higher number of transcripts in LGP32-injected oysters (P < 0.1) (Fig. 7). Interestingly, ΔompU-injected oysters did not differ significantly from the SSW-injected controls (Fig. 7). That this lack of response is caused by OmpU-expression is indicated by the statistical difference observed for intersectin-1 and fascin between LGP32- and ΔompU-injected oysters (P < 0.1). Although calreticulin responded similarly, the response to an injection by the wild-type or the ΔompU mutant was not statistically different (P = 0.16) (Fig. 7). Taken together, these data show that OmpU activates the expression of genes involved in cell remodeling. Conversely, the expression of Cg-EcSOD and β-integrin, which mediate recognition, was not statistically different between LGP32- and ΔompU-injected oysters (Fig S4).
Fig. 7.
OmpU-dependent induction of hemocyte recognition and cell-trafficking genes in experimentally infected oysters. Hemocyte gene expression in oysters injected with LGP32, ΔompU, or SSW was monitored by qPCR. Relative expression was calculated by normalization to C. gigas RPL40. Results are expressed as mean values ± SD (whiskers) and ± SE (boxes). Significant differences between conditions (P < 0.1) were determined by the ANOVA-Kruskal-Wallis test and are indicated by different lowercase letters (a or b). The absence of significant difference is indicated by the use of identical lowercase letters; a,b is used when a condition is neither different from a nor from b.
Discussion
Results showed that V. splendidus LGP32 is a facultative intracellular pathogen that uses OmpU to attach and invade oyster hemocytes through opsonisation by the plasma extracellular superoxide dismutase, Cg-EcSOD. Although usually considered as extracellular pathogens, several Vibrio species support intracellular stages: this was shown for the coral pathogen Vibrio shiloi, which invades epithelial cells (36), and for the human pathogen V. cholerae, which is endocytosed by amoebas (37) and phagocytes in which it retains viability (38). That Vibrio species can have intracellular stages is also supported by the emerging evidence that most pathogenic microbes are capable of intracellular survival, at least during some stages of the infection and disease cycle. Hence, Casedevall proposed that the capacity for intracellular life may be the rule rather than the exception (39).
The major role of hemocyte invasion in the pathology of LGP32 is shown here by the impaired ability of an avirulent mutant (6) to invade oyster hemocytes (Fig. 4). Hemocyte invasion, which was observed both in vivo and in vitro, had deleterious consequences on oyster cellular defenses. Indeed, the presence of viable and culturable intracellular LGP32 was associated with the lack of acidic vacuole formation and an attenuated ROS production, which were clearly observed upon phagocytosis and elimination of the avirulent LMG20012T (Fig. 2). Whether those mechanisms rely on the intracellular secretion of bacterial effectors remains to be established. Such an intracellular secretion was recently shown for type VI secretion (T6SS) effectors of V. cholerae (38), which are also present in the genome of LGP32 (40). The lack of acidic vacuole formation (Fig. 2), indicative of defective phagolysosome maturation, is a mechanism of immune evasion for intracellular pathogenic bacteria like Mycobacterium tuberculosis and Salmonella typhimurium (41), which greatly differs from that of Vibrio aestuarianus, another pathogen associated to the major episodes of mortality in oysters. Indeed, V. aestuarianus avoids phagocytosis by the secretion of inhibitory extracellular products (42), whereas LGP32 uses phagocytosis as part of its pathogenic process (this study). This process is actually more similar to that of a third oyster pathogen, the parasite P. marinus, which invades oyster hemocytes but fails to elicit an oxidative response (27).
OmpU was found here to serve as an adhesin for hemocyte recognition and cell entry. Such a role in host-cell recognition was recently reported in other Vibrio species (14, 15). Like OmpU, OMPs of intracellular pathogens can mediate adherence to host cells, stimulating host transduction pathways required for bacterial entry. One remarkable example is that of OpC from Neisseria meningitidis, which binds to human serum factors like activated vitronectin and to a lesser extent fibronectin to attach to and invade human brain endothelial cells (11). In these cells, fibronectin/vitronectin serve as molecular bridges between the pathogen and the host RGD-recognizing αvβ3-integrin receptors. As previously shown for fibronectin (6, 14), the major protein of oyster plasma, known as Cg-EcSOD, was shown here to specifically recognize OmpU. From this study, Cg-EcSOD mediates attachment to and invasion of hemocytes, serving as an opsonin that promotes phagocytosis of LGP32 (Fig. 4). This process provides the multifunctional Cg-EcSOD with another immune function, the protein also having LPS-binding, iron-binding, and antioxidant properties (19, 43). Binding of Cg-EcSOD to OmpU-expressing LGP32 was dependent on the RGD sequence (Fig. 5B), which is found in both fibronectin and Cg-EcSOD (19) and was reported to mediate the interaction of OmpU from V. vulnificus with fibronectin (14). Thus, as fibronectin binds to OpC in N. meningitidis, Cg-EcSOD binds to OmpU in LGP32 and mediates its interaction with C. gigas host cells.
We showed here that the OmpU-dependent hemocyte invasion by LGP32 is β-integrin–mediated (Fig. 3). This finding is consistent with the identification of β-integrins as major targets for adhesin-dependent phagocytosis, being particularly prone to hijacking by invasive bacteria (44). Integrin-mediated invasion by binding of host major plasma proteins to OMPs has actually been documented in various intracellular pathogens [e.g., N. meningitidis (11), Orientia tsutsugamushi (45) and Moraxella catarrhalis (46)]. Experimental infections of oysters showed that LGP32 signals cytoskeletal remodeling in an OmpU-dependent manner. Indeed, genes like calreticulin, intersectin-1, and fascin, which mediate recognition, clathrin-dependent endocytosis, and actin polymerization are overexpressed in hemocytes from animals injected with wild-type but not ΔompU LGP32 (Fig. 7). These in vivo data support the OmpU-promoted subversion of oyster hemocyte actin cytoskeleton as a major step in the infectious process of LGP32.
Taken together, the data from the present study unravel the cellular and molecular mechanisms associated to the OmpU-dependent virulence of LGP32 evidenced in our previous study (6). We propose a model of hemocyte invasion by LGP32 in which LGP32 is recognized by β-integrin at the surface of oyster hemocytes through an OmpU-specific opsonisation by Cg-EcSOD (Fig. S5). Intracellular bacteria then evade hemocyte defenses (antimicrobial peptides and ROS) by avoiding acidic vacuole formation and limiting the production of ROS. Whether oyster hemocytes are the final target of LGP32 or whether they serve as vehicles for infection of tissues remains to be established. Aside from the present model, the increasing number of reports identifying OmpU as a key mediator of pathogenic interactions indicates that OmpU cell-adhesive and -invasive properties could be of prime importance in many biological models of infections, including human diseases, where fibronectin could serve as a bridging molecule instead of Cg-EcSOD between the pathogen and the host-cell β-integrins.
Materials and Methods
Bacterial Strains.
Vibrio strains (Table 1) were grown at 20 °C in Zobell medium. When needed, 12.5 μg/mL chloramphenicol (Cm) was added. GFP-expressing derivatives of LGP32 and its ΔompU isogenic mutant were obtained by allelic exchange as described previously (6). The gfp gene under the PTRC promoter was integrated in a non essential transposase gene of LGP32 using the pSW3654T suicide vector (Table 1). Primers for pSW3654T construction were Tr32s-Pst1 and Tr32as-BamH1 (Table 1).
Table 1.
Strains, plasmids, and oligonucleotides
| Strains, plasmids, or oligonucleotides | Description or sequence | Reference |
| Bacterial strains | ||
| LGP32 | V. splendidus LGP32 | (1) |
| ΔompU | V. splendidus LGP32 ΔompU | (6) |
| LMG20012T | V. tasmaniensis (V. splendidus-related) | (28) |
| Plasmids | ||
| pSW3654T | pSW23T::PTRC-gfp-Tr32; oriVR6Kγ-oriTRP4 (Cmr) | Present study |
| Oligonucleotides | ||
| Tr32s-Pst1 | 5′-GCCCCTGCAGCCCTAACAAACGCTTCAAGAGGG-3′ | |
| Tr32as-BamH1 | 5′-GCCCGGATCCGTATGAAAGAACGACTCCACCTCCGC-3′ | |
| Cg-fascin F | 5′-CATGTAAAACTGTTGTAGCC-3′ | |
| Cg-fascin R | 5′-ACTCCACATCACTATAACTG-3′ | |
| Cg-intersectin-1F | 5′-AAGTGATCCGTACTGTGAGG-3′ | |
| Cg-intersectin-1R | 5′-GGTCCTTGATTGTGAACTGC-3′ | |
| Cg-calreticulin F | 5′-ACTGGGATGACGAGATGGAC-3′ | |
| Cg-calreticulin R | 5′-GCCAAAGATCAAATCCAACG-3′ | |
| Cg-β-actin F | 5′-CCATGTACGTCGCCATCCAG-3′ | |
| Cg-β-actin R | 5′-GATCACGTCCAGCGAGATCC-3′ | |
| Cg-RPL40 F | 5′-AATCTTGCACCGTCATGCAG-3′ | |
| Cg-RPL40 R | 5′-AATCAATCTCTGCTGATCTGG-3′ |
Animals and Hemolymph Collection.
Adult diploid C. gigas were purchased from local oyster farms in Mèze and Palavas-les-Flots (Gulf of Lion, France). Hemolymph was collected from the posterior adductor muscle sinus using a 2-mL syringe equipped with a 23-G needle. Cell-free hemolymph (plasma) was obtained by centrifugation (3,000 × g, 15 min, 4 °C) and filtration through a 0.22-μm pore-size filter.
Oyster Bacterial Challenge.
Experimental infections were performed at 20 °C, as previously described (6). Oysters were injected with 1 × 108 cfu per animal of wild-type or ΔompU LGP32. Control animals were injected with SSW. For every condition, oysters were placed for 24 h in three separate in 50-L tanks of sea water. For gene-expression analysis, hemolymph was collected from 30 oysters per experimental conditions (10 oysters per tank). Hemocytes were collected from pools of 10 hemolymphs (one pool per tank) by centrifugation (15 min, 1,000 × g, 4 °C) and directly processed for RNA extraction. For confocal microscopy, hemolymph was collected 1 h after injection of GFP-expressing LGP32 (1 × 108 cfu).
Hemocyte Invasion Assays.
GFP-labeled LGP32 in stationary phase of growth and oyster hemolymph or hemocytes were incubated in a 50:1 ratio. When needed, a 1-h preincubation was performed with either plasma, purified Cg-EcSOD, or various inhibitors, including sucrose (Fluka), cytochalasin D (Sigma), 0.4 mg/mL of RGD or EGF tripeptides (Sigma), or 8 μg/mL of anti–β-integrin antibody (generous gift from Marie-Christine Lebart, University of Montpellier 2, Montpellier, France). After fixation with 3.7% formaldehyde, hemocytes were washed twice with SSW and counter stained with Evan's blue (Sigma), Alternatively, hemocytes were stained with phalloidin-tetramethylrhodamine B isothiocyanate and DAPI (Sigma). Hemocyte invasion was monitored after a 30-min contact by confocal microscopy, and after 2 h by flow cytometry.
Confocal Microscopy.
Images were acquired on a Leica SPE confocal laser scanning system connected to a Leica DM 2500 upright microscope. Lasers were used at λex 488 nm for GFP (λem 505–530 nm), λex 532 nm for rhodamine-phalloidin (λem 560–630 nm), λex 405 nm for DAPI (λem 420–480 nm), and λex 405 nm for Evan's blue (λem 670–750 nm). A 40× ACS APO 1.15 oil Leica objective was used. Images were collected sequentially to avoid cross-contamination between fluorochromes and scanned at a 1,024 × 1,024 pixel resolution. Series of optical sections were collected.
Flow Cytometry.
The green fluorescence of LGP32 in hemocytes was monitored in a FACscan apparatus using a 488-nm argon-ion laser. Hemocyte invasion was monitored using at least a total of 30,000 events. Data were analyzed with the Cell Quest software.
Viability of Intracellular Bacteria.
Monolayers of 2.5 × 105 hemocytes were exposed to LGP32 or LMG20012T in a 50:1 ratio. After 1, 4, and 16 h, extracellular bacteria were eliminated with trypsin-EDTA 0.02% and hemocytes were lysed in 0.05% Triton X-100 before plating on Zobell medium for cfu counting.
Quantification of Hemocyte Responses.
Hemocyte suspensions exposed for 1h to LGP32 or LMG20012T in a 50:1 ratio were treated with 0.1 mM green-lysotracker (Invitrogen) or 1 mM DCFH-DA (Sigma). Fluorescence indicative of acidic vacuole formation and ROS production was monitored on a Tecan microplate reader at λex 504/λem 511 and λex 492/λem 530, respectively. Unchallenged hemocytes were used as controls.
Plasma Fractionation.
Pooled plasma from 10 oysters was acidified to pH 3.8 with 2 M HCl. Proteins (2 mg) were separated on a UP5NEC-25QS HPLC column (Interchim) equilibrated in 0.05% trifluoroacetic acid (TFA). Fractionation was performed with a linear gradient of 0 to 70% acetonitrile (ACN) in TFA 0.05% over 35 min at a flow rate of 0.7 mL/min. Absorbance was monitored at 225 nm. Fractions were lyophilized and reconstituted in PBS before SDS/PAGE analysis or binding assays.
LC-MS/MS Analysis.
Protein bands were excised from SDS/PAGE, dehydrated, and incubated with 0.15 μg trypsin (Promega) in 25 mM NH4HCO3 for 16 h at 37 °C. Peptides were extracted from gel pieces on a robot (EVO150; Tecan) with three sequential 30 μL-extractions in 50% ACN, 5% formic acid, and 100% ACN. Peptide extracts were pooled and dried under vacuum before LC-MS/MS analysis on an Ultimate 3000 HPLC (Dionex) coupled with a LTQ-ORBITRAP discovery (Thermo Fisher Scientific) mass spectrometer. The MS-MS sequenced peptides were analyzed by the Mascot search engine for protein identification, against the 29,745 unique ESTs available for C. gigas (http://public-contigbrowser.sigenae.org:9090/Crassostrea_gigas/index.html) and the NCBI database (http://www.ncbi.nlm.nih.gov).
Plasma- and Cg-EcSOD-Binding Assay.
Plasma, HPLC fractions, or purified Cg-EcSOD were coated on a microplate for 16 h at 4 °C. Bound proteins were then overlaid for 1 h at room temperature with GFP-expressing bacteria in stationary phase of growth (108 per well). In specific assays, bacteria were preincubated for 30 min with 400 μg/mL RGD or EGF tripeptides (Sigma) before overlay. Unbound bacteria were washed off with SSW before GFP reading (λex 395 nm/λem 509 nm) on a Tecan Infinite200 microplate reader.
RNA Isolation and qPCR Analysis of Oyster Gene Expression.
RNAs were extracted in TRIzol (Invitrogen) from oyster hemocytes (three pools of 10 oysters per condition). Complementary DNA was synthesized with M-MLV reverse transcriptase (Invitrogen) and diluted for qPCR reactions with LightCycler 480 master mix (Roche). Primers used for intersectin-1 (GenBank CU995003), fascin (GenBank FP007130), calreticulin (GenBank CU993006), and β-actin (GenBank EW779066) are listed in Table 1. Quantitative PCR were carried out in triplicates on the LightCycler 480 System (Roche). Relative expression was calculated using the 2-ΔΔCq method (47), with normalization to the C. gigas RPL40 (GenBank FP004478). Statistics used the nonparametric ANOVA-Kruskal-Wallis test (STATISTICA software).
Acknowledgments
We thank Agnès Vergnes, Julie Fievet, and Marc Leroy for technical assistance, and Dr. Jean-Loup Lemesre (Institut de Recherche pour le Développement, Montpellier) for access to the flow cytometry facilities, and the Montpellier RIO Imaging platform of -University of Montpellier 2 for the confocal microscopy access. This work was supported in part by graduate scholarship fellowships from Institut Français de Recherche pour l'Exploitation de la Mer (Ifremer) (to M.D.), Becas Chile-Comisión Nacional de Investigación Científica y Tecnológica (to P.S.), Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brazil (to R.D.R.), and by Ifremer, the Centre National de la Recherche Scientifique, and the Languedoc-Roussillon Region [“chercheur(se) d'avenir” REVAResp project].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015326108/-/DCSupplemental.
References
- 1.Gay M, Berthe FC, Le Roux F. Screening of Vibrio isolates to develop an experimental infection model in the Pacific oyster Crassostrea gigas. Dis Aquat Organ. 2004;59(1):49–56. doi: 10.3354/dao059049. [DOI] [PubMed] [Google Scholar]
- 2.Gay M, Renault T, Pons AM, Le Roux F. Two Vibrio splendidus related strains collaborate to kill Crassostrea gigas: Taxonomy and host alterations. Dis Aquat Organ. 2004;62(1–2):65–74. doi: 10.3354/dao062065. [DOI] [PubMed] [Google Scholar]
- 3.De Decker S, et al. Responses of diploid and triploid Pacific oysters Crassostrea gigas to Vibrio infection in relation to their reproductive status. J Invertebr Pathol. 2010 doi: 10.1016/j.jip.2010.09.003. 10.1016/j.jip.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Le Roux F, Binesse J, Saulnier D, Mazel D. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl Environ Microbiol. 2007;73:777–784. doi: 10.1128/AEM.02147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binesse J, et al. Metalloprotease vsm is the major determinant of toxicity for extracellular products of Vibrio splendidus. Appl Environ Microbiol. 2008;74:7108–7117. doi: 10.1128/AEM.01261-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duperthuy M, et al. The major outer membrane protein OmpU of Vibrio splendidus contributes to host antimicrobial peptide resistance and is required for virulence in the oyster Crassostrea gigas. Environ Microbiol. 2010;12:951–963. doi: 10.1111/j.1462-2920.2009.02138.x. [DOI] [PubMed] [Google Scholar]
- 7.Negm RS, Pistole TG. The porin OmpC of Salmonella typhimurium mediates adherence to macrophages. Can J Microbiol. 1999;45:658–669. [PubMed] [Google Scholar]
- 8.Weiser JN, Gotschlich EC. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991;59:2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soulas C, et al. Outer membrane protein A (OmpA) binds to and activates human macrophages. J Immunol. 2000;165:2335–2340. doi: 10.4049/jimmunol.165.5.2335. [DOI] [PubMed] [Google Scholar]
- 10.Virji M, Makepeace K, Moxon ER. Distinct mechanisms of interactions of Opc-expressing meningococci at apical and basolateral surfaces of human endothelial cells; The role of integrins in apical interactions. Mol Microbiol. 1994;14(1):173–184. doi: 10.1111/j.1365-2958.1994.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 11.Sa E Cunha C, Griffiths NJ, Virji M. Neisseria meningitidis Opc invasin binds to the sulphated tyrosines of activated vitronectin to attach to and invade human brain endothelial cells. PLoS Pathog. 2010;6:e1000911. doi: 10.1371/journal.ppat.1000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathur J, Davis BM, Waldor MK. Antimicrobial peptides activate the Vibrio cholerae sigmaE regulon through an OmpU-dependent signalling pathway. Mol Microbiol. 2007;63:848–858. doi: 10.1111/j.1365-2958.2006.05544.x. [DOI] [PubMed] [Google Scholar]
- 13.Mathur J, Waldor MK. The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides. Infect Immun. 2004;72:3577–3583. doi: 10.1128/IAI.72.6.3577-3583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goo SY, et al. Identification of OmpU of Vibrio vulnificus as a fibronectin-binding protein and its role in bacterial pathogenesis. Infect Immun. 2006;74:5586–5594. doi: 10.1128/IAI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ Microbiol. 2009;11:483–493. doi: 10.1111/j.1462-2920.2008.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moné Y, et al. A large repertoire of parasite epitopes matched by a large repertoire of host immune receptors in an invertebrate host/parasite model. PLoS Negl Trop Dis. 2010;4(9) doi: 10.1371/journal.pntd.0000813. e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger E, et al. Controlled chaos of polymorphic mucins in a metazoan parasite (Schistosoma mansoni) interacting with its invertebrate host (Biomphalaria glabrata) PLoS Negl Trop Dis. 2008;2:e330. doi: 10.1371/journal.pntd.0000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terahara K, et al. Differences in integrin-dependent phagocytosis among three hemocyte subpopulations of the Pacific oyster “Crassostrea gigas”. Dev Comp Immunol. 2006;30:667–683. doi: 10.1016/j.dci.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez M, et al. Evidence in oyster of a plasma extracellular superoxide dismutase which binds LPS. Biochem Biophys Res Commun. 2005;338:1089–1097. doi: 10.1016/j.bbrc.2005.10.075. [DOI] [PubMed] [Google Scholar]
- 20.Bachère E, et al. In vitro chemiluminescence studies of marine bivalve defence mechanisms and responses against specific pathogens. Dev Comp Immunol. 1991;15:S102. [Google Scholar]
- 21.Anderson RS. Hemocyte-derived reactive oxygen intermediate production in four bivalve mollusks. Dev Comp Immunol. 1994;18:89–96. doi: 10.1016/0145-305x(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez M, et al. Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gigas Cg-BPI. Proc Natl Acad Sci USA. 2007;104:17759–17764. doi: 10.1073/pnas.0702281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt P, Gueguen Y, Desmarais E, Bachère E, de Lorgeril J. Molecular diversity of antimicrobial effectors in the oyster Crassostrea gigas. BMC Evol Biol. 2010;10:23. doi: 10.1186/1471-2148-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt P, et al. Insight into invertebrate defensin mechanism of action: Oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J Biol Chem. 2010;285:29208–29216. doi: 10.1074/jbc.M110.143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasumi S, Vasta GR. A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J Immunol. 2007;179:3086–3098. doi: 10.4049/jimmunol.179.5.3086. [DOI] [PubMed] [Google Scholar]
- 26.Volety AK, Chu FLE. Suppression of chemiluminescence of Eastern oyster (Crassostrea virginica) hemocytes by the protozoan parasite Perkinsus marinus. Dev Comp Immunol. 1995;19(2):135–142. doi: 10.1016/0145-305x(94)00059-o. [DOI] [PubMed] [Google Scholar]
- 27.Schott EJ, Pecher WT, Okafor F, Vasta GR. The protistan parasite Perkinsus marinus is resistant to selected reactive oxygen species. Exp Parasitol. 2003;105:232–240. doi: 10.1016/j.exppara.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Thompson FL, Thompson CC, Swings J. Vibrio tasmaniensis sp. nov., isolated from Atlantic salmon (Salmo salar L.) Syst Appl Microbiol. 2003;26(1):65–69. doi: 10.1078/072320203322337326. [DOI] [PubMed] [Google Scholar]
- 29.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scotti PD, Dearing SC, Greenwood DR. Characterisation of cavortin, the major haemolymph protein of the Pacific oyster (Crassostrea gigas) N Z J Mar Freshw Res. 2007;41(1):91–101. [Google Scholar]
- 31.Petersen SV, et al. The dual nature of human extracellular superoxide dismutase: One sequence and two structures. Proc Natl Acad Sci USA. 2003;100:13875–13880. doi: 10.1073/pnas.2436143100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer DA. Coupling actin dynamics and membrane dynamics during endocytosis. Curr Opin Cell Biol. 2002;14(1):76–81. doi: 10.1016/s0955-0674(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 33.Courson DS, Rock RS. Actin cross-link assembly and disassembly mechanics for alpha-actinin and fascin. J Biol Chem. 2010;285:26350–26357. doi: 10.1074/jbc.M110.123117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: Ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41(2–3):173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 36.Banin E, et al. Penetration of the coral-bleaching bacterium Vibrio shiloi into Oculina patagonica. Appl Environ Microbiol. 2000;66:3031–3036. doi: 10.1128/aem.66.7.3031-3036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abd H, Saeed A, Weintraub A, Nair GB, Sandström G. Vibrio cholerae O1 strains are facultative intracellular bacteria, able to survive and multiply symbiotically inside the aquatic free-living amoeba Acanthamoeba castellanii. FEMS Microbiol Ecol. 2007;60(1):33–39. doi: 10.1111/j.1574-6941.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma AT, Mekalanos JJ. In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc Natl Acad Sci USA. 2010;107:4365–4370. doi: 10.1073/pnas.0915156107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casadevall A. Evolution of intracellular pathogens. Annu Rev Microbiol. 2008;62:19–33. doi: 10.1146/annurev.micro.61.080706.093305. [DOI] [PubMed] [Google Scholar]
- 40.Le Roux F, et al. Genome sequence of Vibrio splendidus: An abundant planctonic marine species with a large genotypic diversity. Environ Microbiol. 2009;11:1959–1970. doi: 10.1111/j.1462-2920.2009.01918.x. [DOI] [PubMed] [Google Scholar]
- 41.Flannagan RS, Cosío G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 42.Labreuche Y, Soudant P, Gonçalves M, Lambert C, Nicolas JL. Effects of extracellular products from the pathogenic Vibrio aestuarianus strain 01/32 on lethality and cellular immune responses of the oyster Crassostrea gigas. Dev Comp Immunol. 2006;30:367–379. doi: 10.1016/j.dci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Itoh N, et al. Characterization of the major plasma protein of the Eastern oyster, Crassostrea virginica, and a proposed role in host defense. Comp Biochem Physiol B Biochem Mol Biol. 2011;158(1):9–22. doi: 10.1016/j.cbpb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Dupuy AG, Caron E. Integrin-dependent phagocytosis: Spreading from microadhesion to new concepts. J Cell Sci. 2008;121:1773–1783. doi: 10.1242/jcs.018036. [DOI] [PubMed] [Google Scholar]
- 45.Cho BA, Cho NH, Seong SY, Choi MS, Kim IS. Intracellular invasion by Orientia tsutsugamushi is mediated by integrin signaling and actin cytoskeleton rearrangements. Infect Immun. 2010;78:1915–1923. doi: 10.1128/IAI.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spaniol V, Heiniger N, Troller R, Aebi C. Outer membrane protein UspA1 and lipooligosaccharide are involved in invasion of human epithelial cells by Moraxella catarrhalis. Microbes Infect. 2008;10(1):3–11. doi: 10.1016/j.micinf.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]