Abstract
Because only a small fraction of asbestos-exposed individuals develop malignant mesothelioma1, and because mesothelioma clustering is observed in some families1, we searched for genetic predisposing factors. We discovered germline mutations in BAP1 (BRCA1-associated protein 1) in two families with a high incidence of mesothelioma. Somatic alterations affecting BAP1 were observed in familial mesotheliomas, indicating biallelic inactivation. Besides mesothelioma, some BAP1 mutation carriers developed uveal melanoma. Germline BAP1 mutations were also found in two of 26 sporadic mesotheliomas: both patients with mutant BAP1 were previously diagnosed with uveal melanoma. Truncating mutations and aberrant BAP1 expression were common in sporadic mesotheliomas without germline mutations. These results reveal a BAP1-related cancer syndrome characterized by mesothelioma and uveal melanoma. We hypothesize that other cancers may also be involved, and that mesothelioma predominates upon asbestos exposure. These findings will help identify individuals at high risk of mesothelioma who could be targeted for early intervention.
Malignant mesotheliomas are aggressive tumors resistant to current therapies2 and associated primarily with widespread commercial use of asbestos in the 20th century1,3-8. Approximately 27 million U.S. workers were exposed to asbestos from 1940-1979, and more thereafter4. In the U.S., mesothelioma incidence in different states varies from 1-2/106 to 15/106 depending on quantities of asbestos used, with ~3,000 deaths/year1. Despite asbestos abatement efforts, mesothelioma rates have remained stable in the U.S. since 1994 and will increase by 5-10% per year in Europe over the next 25 years1,5-7. A marked increase in mesothelioma is predicted in developing countries, where asbestos usage is increasing exponentially8. Erionite shares physical characteristics with asbestos and also causes mesothelioma1. With increased urban development, exposure also occurs from disturbing asbestos- and erionite-containing soil9,10.
Some individuals develop mesothelioma following exposure to small amounts of asbestos, while others exposed to heavy amounts do not1. We have reported mesothelioma clustering in some U.S. and Turkish families in which up to 50% of members developed mesothelioma11. This incidence far exceeds that observed in cohorts exposed to very high levels of asbestos (4.6%1).
Over the past 14 years, we studied prospectively high-risk mesothelioma families to identify a putative mesothelioma-susceptibility gene(s). We focused on two U.S. mesothelioma families, one in Wisconsin (W family) and one in Louisiana (L family), in which members were neither exposed to erionite nor had occupational exposure to asbestos, thus removing the confounding factor of heavy exposure to carcinogens known to cause a high incidence of mesothelioma. Family members developed various malignancies, although mesothelioma predominated (Fig. 1a,b and Supplementary Table 1).
Figure 1.
Pedigrees of two U.S. mesothelioma families. (a, b) Pedigrees showing family members with a germline mutation in BAP1, as confirmed by both sequencing and linkage analyses (orange) or by linkage analysis alone (yellow, i.e., no DNA was available for sequencing); individuals without the mutation (green) and individuals for whom DNA was unavailable (blue) are also shown. Presence or absence of germline BAP1 mutation is also indicated with + or − symbols, respectively. (a) Pedigree of family W showing presence or absence of germline mutation at BAP1 consensus splice acceptor site. (b) Pedigree of family L showing presence or absence of germline nonsense mutation. The development of other tumor types (Supplementary Table 1) in these families may also be related to BAP1 germline mutations. In family W, the presence of a breast cancer before age 45 and an ovarian cancer suggests that the BAP1 mutation is associated with a hereditary form of breast/ovarian cancer, as might be expected given BAP1’s relationship with the breast/ovarian cancer susceptibility gene product, BRCA115. In family L, the skin cancers shown were squamous cell carcinomas. Available mesothelioma tumor specimens with germline splice site mutation and either somatic 25-bp deletion (W-III-04T), genomic alteration (W-III-06T), or loss of wild-type BAP1 allele (W-III-08T) are indicated in Supplementary Table 1, as is the homozygous deletion of BAP1 seen in mesothelioma specimen L-III-18T.
Array-CGH analysis of two tumors (one/family) uncovered alterations encompassing or adjacent to the BAP1 locus in 3p21.1. In one tumor, W-III-06T (T=tumor), a transition in copy number occurred at or near the BAP1 promoter, while in the second, L-III-18T, a focal deletion encompassing BAP1 resided within a larger deletion (Fig. 2a). We also performed linkage studies on germline DNA from each family. The largest linkage peak, reaching a maximum LOD score of 2.1, in a joint parametric analysis of the two families occurred at 3p21-22 (Supplementary Fig. 1). Although the region implicated in linkage analyses assuming that only those with mesothelioma were affected was large and included many genes, a much smaller region was implicated by the array-CGH analysis, including a genomic imbalance beside the BAP1 locus and an ~218-kb homozygous deletion that encompassed BAP1. Moreover, this smaller region was congruent with a smaller linkage region obtained in the W family assuming that all those with cancer, including kidney, ovary and early onset breast carcinomas, carried the same risk allele.
Figure 2.
Array-CGH analysis of two mesothelioma family members and schematic diagrams of predicted mutant BAP1 proteins. (a) Array-CGH showing focal deletion encompassing BAP1 within a larger 3p deletion (tumor L-III-18T) and amplicon adjacent to BAP1 locus (tumor W-III-06T). The BAP1 gene resides at chr3:52,435,027-52,444,009, and the Agilent Human 244K chip contains two probes within the BAP1 locus: A_16_P00704764 (chr3:52,438,014-52,438,066) and A_14_P128339 (chr3:52,443,209-52,443,268). In W-III-06T, the two BAP1 probes displayed log2 ratios indicative of normal diploid DNA copy numbers, whereas the log2 ratios of two probes immediately centromeric of BAP1 showed a transition to a higher copy number, indicating the start of an amplified region at or very near the BAP1 promoter (zoomed-in image and further details shown in Supplementary Fig. 6). In L-III-18T, the focal homozygous deletion encompassed the entire BAP1 locus and was ~218 kb in size. (b) Schematic diagram of predicted truncations of BAP1 resulting from germline mutations observed in two mesothelioma families (W, L) as well as in two sporadic mesothelioma patients who previously had developed uveal melanomas (SP-002; SP-008).
These findings, coupled with the fact that 3p21.1 is a site of recurrent chromosomal loss in sporadic mesotheliomas12-14 prompted our pursuit of this gene. BAP1 is a nuclear protein that enhances BRCA1-mediated inhibition of breast cancer cell proliferation, acting as a tumor suppressor in the BRCA1 growth control pathway and regulating proliferation by deubiquitinating host cell factor-115,16.
We sequenced BAP1 in germline DNA from family W and found that six affected members (four with mesothelioma; two with breast or renal cancer) had identical mutations, whereas unaffected family members did not (Fig. 1a). Mutation status was consistent with results of the linkage analysis, the latter additionally establishing that case W-III-10 (ovarian cancer) was a BAP1 mutation carrier. The germline mutation in family W occurred at the intron 6/exon 7 boundary, with affected individuals having an A→G substitution at the -2 nucleotide consensus splice acceptor site (Supplementary Fig. 2a). Such alterations lead to exon skipping and disease-causing protein changes17,18. Transfection of mammalian cells with a genomic construct containing exons 6-8, and with the intron 6 splice site mutation, resulted in an aberrant splice product lacking exon 7 (Supplementary Fig. 2b) and frameshift predicted to lead to a premature stop codon and nonsense-mediated decay.
Besides the somatic genetic alteration detected by array-CGH in sample W-III-06T, in tumor W-III-08T only mutant BAP1 could be detected, consistent with loss of wild-type BAP1 on the other homologue. Additionally, W-III-04T had both the splice site mutation and a 25-bp exon 4 deletion resulting in a frameshift and premature termination of BAP1 (p.I72fsX7) (Supplementary Fig. 2c). Matched germline DNA lacked the deletion, indicating a somatic origin. Cloning of genomic PCR products encompassing exons 4-7 from tumor DNA suggested that the splice site mutation and deletion reside in different alleles, consistent with biallelic inactivation of BAP1.
In family L, germline DNA from three individuals with mesothelioma (one recently treated for uveal melanoma) and two with skin carcinomas exhibited a germline C/G to T/A transition in exon 16, creating a stop codon (p.Q684X) (Supplementary Fig. 2d). There was complete concordance between BAP1 mutation status and linkage analysis. Linkage analyses also revealed that L-II-03 (pancreatic cancer) was a mutation carrier.
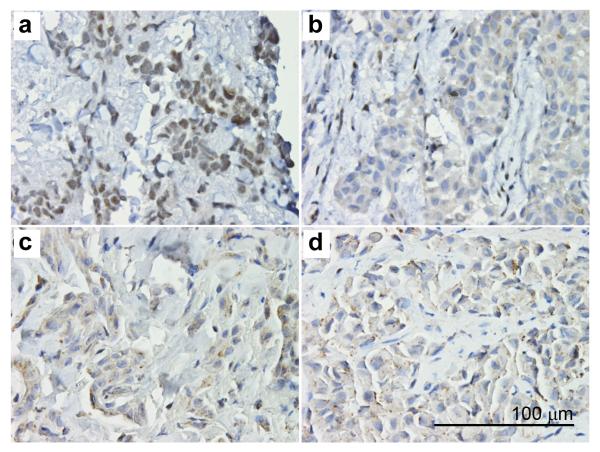
Exome sequencing, using the Illumina HiSeq 2000 system, of germline DNA from two affected members of each family verified the splice site and nonsense mutations in family W and family L, respectively (not shown). Immunohistochemistry on mesotheliomas from L and W families revealed lack of BAP1 nuclear expression (Fig. 3).
Figure 3.
Immunohistochemistry on mesotheliomas from L and W families reveals lack of BAP1 nuclear expression and only weak, focal cytoplasmic BAP1 staining. (a) SP-024, sporadic mesothelioma with wild-type BAP1; note the normal nuclear expression of BAP1. (b) W-III-04, (c) L-III-18, and (d) W-III-06 represent mesotheliomas from patients with germline BAP1 mutations: note lack of nuclear expression and weak cytoplasmic staining. All magnifications 400X. Bar = 100 μm.
Although occupational histories did not suggest any obvious exposure in these families, we detected chrysotile asbestos in 5/5 homes where all affected family L members had lived (consistent with the high percentage of asbestos-containing houses in the town where the family resided); traces of tremolite and chrysotile asbestos were found in the home where all affected members of family W were raised (Supplementary Figs. 3,4). About 30 million U.S. homes contain asbestos4; both families lived in such homes. Living in an asbestos-containing home is associated with modest levels of exposure, as observed in patients who have not been occupationally exposed, a growing fraction of mesothelioma patients1.
Having linked BAP1 mutations to familial mesothelioma, we next sequenced BAP1 (17 exons/introns/promoter) in 26 germline DNAs from sporadic mesothelioma patients. All of them had reported asbestos exposure to the treating physician, although these claims were not verified by lung content or mineralogical analyses. Two of 26 had BAP1 deletions: c.1832delC in exon 13 (p.P572fsX3) and c.2008-2011delTACT in exon 14 (p.Y627fsX9) (Supplementary Table 1). Both mutations result in a frameshift leading to a stop codon upstream of the region encoding the BAP1 nuclear localization signal (Fig. 2b). When we investigated whether anything was unique about these two patients, we found that each had been treated for uveal melanoma 1 or 6 years before being diagnosed with mesothelioma. Of the remaining 24 sporadic mesotheliomas, none had uveal melanoma. Tumor DNA was available from 18 of the 26 sporadic mesothelioma patients: DNA sequencing revealed truncating BAP1 mutations in 4/18 (22%) tumors (Fig. 4a); BAP1 alterations in these tumors were supported by immunoblot analyses (Fig. 4b). Also, 7/12 mesothelioma cell lines tested showed loss of BAP1 expression (Supplementary Fig. 5a). Re-expression of BAP1 in BAP1-deficient mesothelioma cells markedly decreased colony-forming ability (Supplementary Fig. 5b), consistent with BAP1’s known role in regulating tumor cell proliferation and viability15,16.
Figure 4.
BAP1 truncating mutations and aberrant protein expression in sporadic mesothelioma tumor biopsies. (a) Schematic diagram of predicted truncations of BAP1 in four sporadic mesotheliomas harboring BAP1 mutations. Bracket at left indicates mutations in two different BAP1 alleles in tumor sample SP-015. NLS, nuclear localization signal at carboxy-terminus of BAP1. Frameshift sequences are shown as thinner gray bars. (b) Immunoblot analysis on whole tumor cell lysates of the same four sporadic mesotheliomas with somatic BAP1 mutations (lanes 2-5) and sporadic tumor lacking a BAP1 mutation (lane 1). Sporadic mesotheliomas with somatic BAP1 mutations show decreased expression of BAP1 compared to that seen in tumor without BAP1 mutation. Note that in mesotheliomas, whole tumor cell lysates inevitably contain some normal stromal cells that may be responsible for the faint BAP1 signal detected. Also note the presence of additional, faster-migrating BAP1 band in sample shown in lane 4 (SP-013), suggesting the presence of a truncated form of BAP1. The BAP1 protein products predicted in tumors SP-001 and SP-015 were not observed, suggesting nonsense-mediated mRNA decay. The mutation in tumor SP-018 results in a predicted protein product only 15 amino acids smaller, which presumably precludes detection of a small change in molecular weight compared to wild-type BAP1. GAPDH was used as a loading control.
Frequent somatic mutations of BAP1 were reported in metastasizing uveal melanomas, with one case having a germline mutation19. An association between uveal melanoma, breast and ovarian cancer has been proposed20. Neither study uncovered any mesothelioma, possibly because those patients were not exposed to asbestos. A paper published while our manuscript was being reviewed reported somatic BAP1 mutations in 23% of sporadic mesotheliomas21, which concurs with our findings in sporadic tumors. In addition, and most importantly, we demonstrate the presence of germline BAP1 mutations in members of U.S. families that experience an extremely high incidence of mesothelioma, in spite of very modest exposure to asbestos; thus, our results point to BAP1 as the first reported gene that may modulate mineral fiber carcinogenesis. Furthermore, we show that BAP1 mutations are associated with a novel hereditary cancer syndrome that predisposes to mesothelioma, uveal melanoma and potentially other cancers. The incidence of uveal melanoma is 5-7/106 in the U.S.22, similar to mesothelioma1. Therefore, it is exceedingly unlikely that the occurrence of both malignancies in the same individual would occur by chance, e.g., if we assume the two diseases are independent and the joint probability (estimated at 36 per trillion per year) follows a binomial distribution, then the likelihood of three (or more) cases appearing in the U.S. (population ~310 million) per year is 2.3 × 10−7.
The average age of diagnosis of uveal melanoma is 56, with 5-year survivals of 70-99% depending on tumor size and histology22. Mesothelioma is diagnosed later in life, and a 5-year survival is extremely rare and limited to patients diagnosed during the very early stages of tumor growth, when patients can undergo cytoreductive surgery1,2. Altogether, we observed BAP1 mutations in four uveal melanoma patients, three of whom subsequently developed mesothelioma; the fourth (L-II-18) died of metastatic uveal melanoma to the liver (Fig. 1b), and DNA was not available. Thus, our findings suggest that uveal melanoma patients with germline BAP1 mutations are at high risk of developing mesothelioma and should be closely monitored.
Our results provide the first demonstration that genetics influences the risk of mesothelioma, a cancer linked to mineral fiber carcinogenesis. As observed for BRCA1 and BRCA2, which account for only some hereditary breast carcinomas, it appears likely that in addition to BAP1, more genes will be found associated with elevated risk of mesothelioma. Indeed, among our 26 sporadic mesotheliomas – and excluding malignancies common in the 6th-8th decades of life, such as skin and prostate carcinomas – nine had been diagnosed with one or more additional tumors (Supplementary Table 1). Seven of 26 were females, and 2/7 also had uterine leiomyosarcoma, a malignancy with an incidence of ~10/106 per year in the U.S.; one of them had also uveal melanoma, an unlikely coincidence.
In summary, we demonstrate the existence of a BAP1-related cancer syndrome characterized by mesothelioma, uveal melanoma and possibly other cancer types. We hypothesize that when individuals with BAP1 mutations are exposed to asbestos, mesothelioma predominates. Alternatively, BAP1 mutation alone may be sufficient to cause mesothelioma.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturegenetics/.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the affected individuals and their families for their participation in this study. We thank Marco Bianchi, Craig Menges, Ian Pagano, Janet D. Rowley and David C. Ward for advice and review of the manuscript. We also thank Jacqueline Talarchek and Ryan Harrington for technical assistance, Keston Aquino-Michaels for assisting in the collection of samples and clinical information, Suresh C. Jhanwar for providing some of the mesothelioma cell lines, Anil Vachani for blood and tissue samples from one member of the L family, and Biao Luo for providing exome sequence data. This work was supported by NIH grants P01CA-114047, P30CA-06927, and P30CA-71789, by the AACR-Landon Award for International Collaboration in Cancer Research to M.Ca., N.C., H.I.P., J.R.T., M.T. and H.Y., and by the Local No. 14 Mesothelioma Fund of the International Association of Heat and Frost Insulators & Allied Workers to J.R.T.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Accession codes. BAP1 protein mutation nomenclature numbering is derived from accession NP_004647.1
Note: Supplementary information is available on the Nature Genetics website.
Text references
- 1.Carbone M, et al. Malignant mesothelioma: Facts, myths and hypotheses. J. Cell. Physiol. 2011 Mar 16; doi: 10.1002/jcp.22724. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores RM, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J. Thorac. Cardiovasc. Surg. 2008;135:620–626. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 3.Tweedale G. Asbestos and its lethal legacy. Nat. Rev. Cancer. 2002;2:311–315. doi: 10.1038/nrc774. [DOI] [PubMed] [Google Scholar]
- 4.Kamp DW. Asbestos-induced lung diseases: an update. Transl. Res. 2009;153:143–152. doi: 10.1016/j.trsl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009;20:935–944. doi: 10.1007/s10552-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M, et al. The French National Mesothelioma Surveillance Program. Occup. Environ. Med. 2006;63:390–395. doi: 10.1136/oem.2005.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton M. The epidemiology of mesothelioma. Semin. Oncol. 2002;29:18–25. doi: 10.1053/sonc.2002.30237. [DOI] [PubMed] [Google Scholar]
- 8.Burki T. Health experts concerned over India’s asbestos industry. Lancet. 2010;375:626–627. doi: 10.1016/s0140-6736(10)60251-6. [DOI] [PubMed] [Google Scholar]
- 9.Pan XL, Day HW, Wang W, Beckett LA, Schenker MB. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am. J. Respir. Crit. Care Med. 2005;172:1019–1025. doi: 10.1164/rccm.200412-1731OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone M, et al. Erionite exposure in North Dakota and in the Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. USA. 2011 Jul 25; doi: 10.1073/pnas.1105887108. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone M, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat. Rev. Cancer. 2007;7:147–154. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- 12.Flejter WL, Li FP, Antman KH, Testa JR. Recurring loss involving chromosomes 1, 3, and 22 in malignant mesothelioma: possible sites of tumor suppressor genes. Genes Chromosomes Cancer. 1989;1:148–54. doi: 10.1002/gcc.2870010207. [DOI] [PubMed] [Google Scholar]
- 13.Lu YY, Jhanwar SC, Cheng JQ, Testa JR. Deletion mapping of the short arm of chromosome 3 in human malignant mesothelioma. Genes Chromosomes Cancer. 1994;9:76–80. doi: 10.1002/gcc.2870090114. [DOI] [PubMed] [Google Scholar]
- 14.Murthy SS, Testa JR. Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J. Cell. Physiol. 1999;180:150–157. doi: 10.1002/(SICI)1097-4652(199908)180:2<150::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Jensen DE, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 16.Ventii KH, et al. BRCA1-associated protein-1 Is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, et al. Diverse mutations in patients with Menkes disease often lead to exon skipping. Am. J. Hum. Genet. 1994;55:883–889. [PMC free article] [PubMed] [Google Scholar]
- 18.Baralle D, Baralle M. Splicing in action: assessing disease causing sequence changes. J. Med. Genet. 2005;42:737–748. doi: 10.1136/jmg.2004.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbour JW, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houlston RS, Damato BE. Genetic predisposition to ocular melanoma. Eye. 1999;13:43–46. doi: 10.1038/eye.1999.9. [DOI] [PubMed] [Google Scholar]
- 21.Bott M, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat. Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert DM, Van Buren JJ. Intraocular Melanomas. In: De Vita VT, Lawrence TS, Rosenberg SA, editors. Cancer, Principles & Practice of Oncology. Vol. 1. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. pp. 2090–2098. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.