Abstract
Implicit skill learning underlies obtaining not only motor, but also cognitive and social skills through the life of an individual. Yet, the ontogenetic changes in humans’ implicit learning abilities have not yet been characterized, and, thus, their role in acquiring new knowledge efficiently during development is unknown. We investigated such learning across the life span, between 4–85 years of age with an implicit probabilistic sequence learning task, and we found that the difference in implicitly learning high vs. low probability events - measured by raw reaction time (RT) - exhibited a rapid decrement around age of 12. Accuracy and z-transformed data showed partially different developmental curves suggesting a re-evaluation of analysis methods in developmental research. The decrement in raw RT differences supports an extension of the traditional 2-stage lifespan skill acquisition model: in addition to a decline above the age 60 reported in earlier studies, sensitivity to raw probabilities and, therefore, acquiring new skills is significantly more effective until early adolescence than later in life. These results suggest that due to developmental changes in early adolescence, implicit skill learning processes undergo a marked shift in weighting raw probabilities vs. more complex interpretations of events, which, with appropriate timing, prove to be an optimal strategy for human skill learning.
Keywords: skill learning, implicit sequence learning, automaticity, Alternating Serial Reaction Time Task (ASRT), development, aging, critical period
It is widely accepted that children should be introduced to sports, music or languages early in their life if they are to develop a high proficiency, because late learners seldom become true champions or elite musicians or gain command of a second language similar to that of a native speaker. These observations contradict traditional measures of the ability of factual learning of declarative memories, which showed that humans become increasingly better at many learning tasks up until their late twenties (Craik & Bialystok, 2006). However, an important component of developing new abilities is related to implicit unconscious statistical learning processes (Hikosaka, Nakamura, Sakai, & Nakahara, 2002; Keele, Ivry, Mayr, Hazeltine, & Heuer, 2003) that underlie the acquisition of not only motor but also cognitive and social skills (Doyon, et al., 2009; Hikosaka et al., 2002; Lieberman, 2000; Poldrack, et al., 2005; Ullman, 2001). Thus, to understand complex skill acquisition, the characteristics of both explicit declarative and implicit learning, such as the differences in their efficiency across the lifespan, must be clarified. In contrast to declarative memory (Tulving & Craik, 2000), the ontogenetic changes in humans’ implicit learning abilities have not yet been comprehensively characterized, and, thus, their role in acquiring new knowledge efficiently during development is unknown. The main goal of our study was to examine age differences in implicit learning across the human lifespan using the same task for all groups.
The computational underpinnings and the neural substrates of these different kinds of learning mechanisms are also controversial (Henke, 2010). Explicit learning has been linked more closely to medial temporal lobes of the cortex (Dennis & Cabeza, in press; Squire & Zola, 1996). By contrast, implicit skill learning often requires fine-tuning of the perceptual-motor system based on experience; therefore, most models of implicit skill learning emphasize the role of the basal ganglia and the cerebellum (Cohen, Pascual-Leone, Press, & Robertson, 2005; Dennis & Cabeza, in press; Doyon et al., 2009; Hikosaka, et al., 1999; Hikosaka et al., 2002), whereas the role of the hippocampus remains inconclusive (Albouy, et al., 2008; Schendan, Searl, Melrose, & Stern, 2003). However, these models focused mostly on motor skill-related learning with less emphasis on more complex skills that could involve learning abstract cognitive dependencies implicitly. The second goal of our study was to relate our behavioral results to the various computational models of explicit and implicit learning.
Two main approaches to implicit learning emerged in developmental neuroscience with a different assessment of how learning abilities change with age: 1) the developmental invariance model and 2) the age-related changes model. Studies supporting the developmental invariance model of implicit learning failed to find significant age-related differences in learning (Meulemans, Van der Linden, & Perruchet, 1998; Vinter & Perruchet, 2000). In support of this view, infant studies have shown that adult-like implicit learning mechanisms exist even in very early infancy (Clohessy, Posner, & Rothbart, 2001; Saffran, Aslin, & Newport, 1996). Developmental invariance models explain this age-independence by linking implicit (or procedural) learning to evolutionarily primitive brain regions, such as the basal ganglia and the cerebellum. These regions are characterized as early-maturation regions and are relatively resistant to neurological impairments (Reber, 1993).
By contrast, the age-related changes models posit that considerable developmental differences can be observed in implicit learning. Several of these studies found that older children and young adults showed stronger learning effects compared to very young participants (Fletcher, Maybery, & Bennett, 2000; Kirkham, Slemmer, Richardson, & Johnson, 2007; Maybery, Taylor, & O'Brien-Malone, 1995; Thomas, et al., 2004). These models accept the fronto-striatal origin of such learning, but they focus on evidence of continued development of these regions that form the basis of the behavioral changes with age (e.g., Thomas et al., 2004). We compared our empirical results using a new approach to the problem of multiple neural substrates of learning proposed by Daw et al. (2005).
Serial reaction time task and the development of implicit learning
In our study, we used a modified version of the Serial Reaction Time (SRT) Task, which is one of most commonly used methods for measuring implicit skill learning. Serial Reaction Time Task is a four-choice reaction time task containing a hidden repeating sequence that the subject comes to predict and learn implicitly (Nissen & Bullemer, 1987; Poldrack et al., 2005). In an SRT study, Meulemans et al. (1998) found that 6- and 10-year-old children showed similar degrees of learning as young adults. In contrast, Thomas et al. (2004) found that the learning performance of young adults was better than 7- to 10-year-old children. Studies investigating implicit skill learning at older ages also revealed inconsistent results. For example, several studies have demonstrated that, for simple repeating patterns (in the SRT task), the extent of implicit sequence learning in elderly adults was comparable to young adults (Frensch & Miner, 1994; D. V. Howard & Howard, 1992; D. V. Howard & Howard, 1989). Moreover, in a recent study, Gaillard et al. (2009) found that young (22-year-old), middle-aged (45-year-old), and elderly (71-year-old) participants performed at the same level.
The studies mentioned above used fixed (deterministic) sequences, which can be easily learned, making it less possible to detect age-related differences in learning. Furthermore, they cannot purely determine the acquired sequence-specific knowledge because these tasks (finger-tapping, classical SRT) confound general improvements with sequence-specific learning. Here, we used a modified version of the SRT task, the Alternating Serial Reaction Time (ASRT) task (J. H. Howard, Jr. & Howard, 1997), which enabled us to measure the “pure” sequence-specific learning distinguished from general improvements. In the classical SRT task, the structure of a sequence is deterministic with the stimuli following a simple cyclically repeating pattern (e.g., 213412134121341213412…, where numbers refer to distinct events within the repeating 21341 pattern). By contrast, in the ASRT task (J. H. Howard, Jr. & Howard, 1997; Remillard, 2008), repeating events alternate with random ones. Thus, the location of every second stimulus on the screen was determined randomly. If, for instance, the sequence was 12341234…, where the numbers represent locations on the screen, the sequence of stimuli would be 1R2R3R4R1R2R3R4R… in the ASRT paradigm, with R representing a random element. Therefore, the location of every second stimulus on the screen was determined randomly. Because fixed, sequence-specific and random stimuli were alternating, some sequences of three events (called ‘triplets’) occurred more frequently than others. For example, in the above illustration 1×2, 2×3, 3×4 and 4×1 would occur often, whereas 1×3 or 4×2 would occur infrequently. Following previous studies, we referred to the former as high-frequency triplets and the latter as low-frequency triplets (Nemeth, Janacsek, Londe, et al., 2010; Song, Howard, & Howard, 2007). Previous studies have shown that as people practice the ASRT task, they respond more quickly to the high than low frequency triplets, revealing probabilistic, sequence-specific learning (J. H. Howard, Jr. & Howard, 1997; Song et al., 2007). This learning is statistical in nature because it depends on the frequency of the event sequences. Thus, the RT difference between the high and low frequency triplets in this ASRT task is a measure of human sensitivity to the relative raw probabilities of events observed implicitly in their environment (Perruchet & Pacton, 2006). In addition, the participants are not generally aware of the alternating structure of the sequences, even after extended practice, or when sensitive recognition tests are used to assess explicit knowledge (D. V. Howard, et al., 2004; J. H. Howard, Jr. & Howard, 1997; Song et al., 2007). Thus, the ASRT task is more implicit than the classical deterministic sequence learning tasks.
Using the ASRT task, recent studies have shown that, although elderly adults can also learn the higher-order structure of these complex sequences, they showed age-related deficits (D. V. Howard et al., 2004; J. H. Howard, Jr. & Howard, 1997; Nemeth, Janacsek, Londe et al., 2010). Both young and elderly adults were able to learn third-order dependencies (1RR2RR3RR1RR2RR3RR…) although the elderly participants performed at a lower level than the younger participants (Bennett, Howard, & Howard, 2007). Whereas several studies investigated implicit learning in children using the ASRT task (Barnes, et al., 2008; Barnes, Howard, Howard, Kenealy, & Vaidya, 2010; Nemeth, Janacsek, Balogh, et al., 2010), no child-adult comparison of implicit skill learning performance has yet been reported.
In summary, previous studies have addressed the development and aging in implicit skill learning, but no studies have examined age-related differences from childhood to old age with identical methods. Furthermore, in contrast to general skill improvements, using a probabilistic sequence learning task (ASRT) can help us to reveal the age-related differences of the underlying mechanisms of complex skill learning by measuring explicitly the sensitivity to raw probabilities of high and low frequency events. Therefore, in this study, we compared the implicit probabilistic sequence learning across the age range of 4–85 years.
Method
Participants
There were 421 participants in the experiment between the ages of 4 and 85 that were clustered into nine age groups between 4–6, 7–8, 9–10, 11–12, 14–17, 18–29, 30–44, 45–59 and 60–85 years of age (Table 1). None of them suffered from any developmental, psychiatric or neurological disorders. All subjects gave signed informed consent (parental consent was obtained for children), and they received no financial compensation for participation. All experimental procedures were approved by the Ethics Committee of the University of Szeged.
Table 1.
Demographic data and mean RT and accuracy in the different groups. In all columns, numbers in parentheses show standard deviation.
| Group | Age | Sex | Education | Mean RT (ms) |
Mean Accuracy (%) |
|---|---|---|---|---|---|
| 4–6-year-old (n=30) |
5.31 (0.98) | 17 M / 13 F | - | 960.06 (214.67) | 90.09 (6.34) |
| 7–8-year-old (n=55) |
7.09 (0.56) | 31 M / 24 F | 1.18 (0.39) | 773.24 (159.29) | 90.66 (7.03) |
| 9–10-year-old (n=35) |
9.89 (0.58) | 14 M / 21 F | 3.2 (0.96) | 602.84 (121.03) | 93.44 (4.21) |
| 11–12-year-old (n=29) |
11.5 (0.5) | 21 M / 8 F | 4.66 (0.67) | 544.15 (95.00) | 92.52 (4.23) |
| 14–17-year-old (n=62) |
14.89 (1.06) | 46 M / 15 F | 8.23 (1.02) | 452.52 (67.06) | 95.44 (2.92) |
| 18–29-year-old (n=63) |
23.09 (3.67) | 40 M / 23 F | 15.45 (2.6) | 401.79 (50.85) | 95.47 (2.45) |
| 30–44-year-old (n=59) |
35 (4.24) | 24 M/ 35 F | 16.64 (3.1) | 419.85 (58.68) | 95.85 (2.98) |
| 45–59-year-old (n=36) |
50.8 (5.07) | 12 M / 24 F | 14.18 (3.58) | 526.7 (112.99) | 97.4 (3.45) |
| 60–85-year-old (n=52) |
69.85 (6.16) | 16 M / 36 F | 13.39 (3.04) | 634.37 (126.54) | 96.92 (2.38) |
Implicit probabilistic sequence learning task
We used the ASRT task (J. H. Howard, Jr. & Howard, 1997; Nemeth, Janacsek, Londe et al., 2010) where a stimulus (a dog’s head) appeared in one of the four empty circles arranged in a line on a computer screen. The participants were instructed to respond to different stimulus events by pressing the corresponding response keys (Z, C, B or M) as fast and accurately as possible. The ASRT task consisted of 20 blocks with 85 key presses in each block. The first five responses of each stimulus block served for practice only, and then the eight-element alternating sequence (e.g., 1R2R3R4R) was repeated ten times within a block. Stimuli were presented 120 ms after the response to the previous stimulus. Between blocks, the participants received feedback on the screen about their overall reaction time (RT) and accuracy. The computer program generated a different repeating ASRT sequence of the 4 locations for each participant using a permutation rule such that each of the six unique permutations of the 4 repeating events occurred with equal probability.
To determine the amount of explicit knowledge the subjects acquired about the task, a short questionnaire was administered after the experimental session (Song et al., 2007). This questionnaire included increasingly specific questions, such as “Have you noticed anything special regarding the task?”, “Have you noticed some regularity in the sequence of stimuli?”. The experimenter rated subjects’ answers on a 5-point scale where 1 denoted “Nothing noticed” and 5 denoted “Total awareness”. None of the participants, young or old, reported noticing the hidden repeating sequence.
Statistical properties of the ASRT task
As mentioned above, the ASRT allows a comparison between responses to high- and low-probability events. For example, if the eight-element sequence is 1R2R3R4R, 1×2, 2×3, 3×4, and 4×1 would occur often (high frequency triplets) because two consecutive stimuli of the repeating sequence (e.g., 132 consisting 1R2) as well as two consecutive random elements by chance (e.g., the same 132 consisting R3R) could form these triplets. By contrast, 1×3 or 4×2 would occur less frequently (low frequency triplets) because they could never be obtained consisting two consecutive sequence elements. Of the 64 possible triplets, sixteen triplets were high frequency triplets, occurring 62.5% of the time, whereas the remaining 48 triplets were low frequency triplets, occurring 37.5% of the time. Thus, each low frequency triplet occurred in approximately 0.8% of the total number of trials, whereas each high frequency triplet occurred about 5 times more often, in approximately 4% of the trials. For each keypress response, we defined whether it was in response to a high- or a low frequency element, depending on whether the element was more or less predictable based on the previous two items in the sequence.
Following the method of previous studies (D. V. Howard et al., 2004; Song et al., 2007), two kinds of low frequency triplets were excluded from our analyses: repetitions (e.g., 222, 333) and trills (e.g., 212, 343). Repetitions and trills were low frequency for all participants, and in previous studies, the participants often showed pre-existing response tendencies towards them (D. V. Howard et al., 2004; Soetens, Melis, & Notebaert, 2004). The elimination of these special triplets from the analyses ensured that the high versus low frequency differences found in the study were not confounded by pre-existing response tendencies. After this adjustment, previous studies have found that, following the practice, participants responded more quickly to the high than to the low frequency triplets, revealing a sequence learning effect (D. V. Howard et al., 2004; J. H. Howard, Jr. & Howard, 1997; Nemeth, Janacsek, Londe et al., 2010; Song et al., 2007).
Statistical analysis
We calculated the mean accuracy of all trials and the median reaction time (RT) of correct responses separately for high and low frequency triplets. The accuracy and RT measures were analyzed by mixed-model ANOVA with TRIPLET TYPE (high vs. low frequency) as the within-subject factor, and AGE (9 groups) as the between-subject factor. All significant results are reported together with the ηp2 effect size and Greenhouse-Geisser ε correction factors where applicable. Post-hoc analysis was conducted by Fisher’s LSD pairwise comparisons.
Results
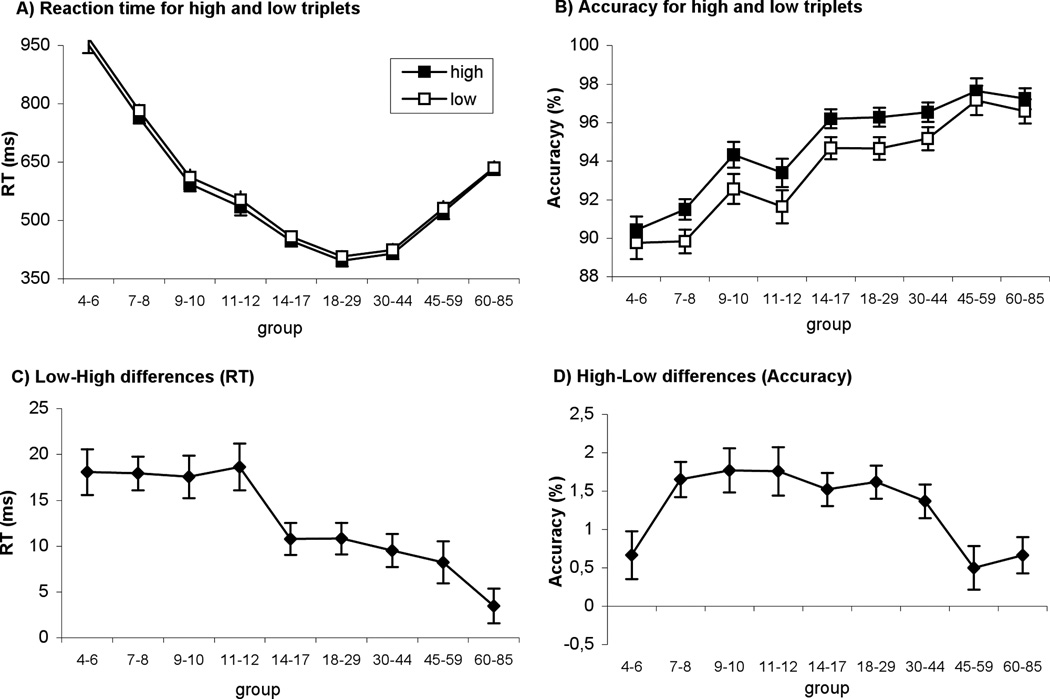
Overall RT’s significantly differed among the age groups (main effect of AGE: F(8,412)=107.11, p<0.001, ηp2=0.675; Table 1). The RT decreased significantly between each group from 4–6 to 18–29 years of age (all p’s<0.04), they were similar between the age groups of 18–29 and 30–44 (p>0.38) and significantly increased after 44 years of age (p’s<0.001) (Figure 1a). The accuracy monotonically increased over the years (main effect of AGE: F(8,412)=16.94, p<0.001, ηp2=0.25) (Figure 1b).
Figure 1.
Sequence learning in all groups. Reaction time (A) and accuracy (B) for high and low frequency triplets are plotted. Learning measures of RT (C) and accuracy (D) represents the RT/ACC difference between low- and high-frequency triplets. Error bars indicate SEM.
The comparison of RT to high vs. low probability triplets showed a surprising pattern of implicit sequence learning across the age groups. Even though there was a significant learning at all ages because the RT’s were faster for high frequency than to low frequency triplets (main effect of TRIPLET TYPE: F(1,412)=333.7, p<0.001, ηp2=0.45, all p’s <0.03) (Figure 1c), the magnitude of this difference was not uniform. Although the age groups differed significantly from each other in sequence learning (TRIPLET TYPE × AGE interaction: F(8,412)=6.79, p<0.001, ηp2=0.12), the post-hoc test revealed that learning was significantly higher in the 4- to 12-year-old groups than in any other group in the 14–85 range (p’s<0.02). There was no difference in learning between the 14–59 years of age (p’s>0.37), whereas the magnitude of learning decreased significantly in the 60–85-year-old group (p’s<0.02). Thus, learning high probability events was uniformly effective until the age of 12 where it reduced significantly and remained at a lower level of sensitivity until the age of 60 (Figure 1c).
However, it is a long-standing issue in developmental and aging studies how to compare groups with different baseline speeds. A customary approach to this problem is to analyze the data using z-transformation. Therefore, we calculated the z-scores within each subject (thus, each participant's own mean and SD was used to transform that participant's data (see, for example, Christ, White, Mandernach, & Keys, 2001) and conducted an ANOVA based on these z-scores (Figure 2). ANOVA revealed significant sequence-specific learning (main effect of TRIPLET TYPE: F(1,412)=320.12, p<0.001, ηp2=0.44), but the extent of learning differed across groups (TRIPLET TYPE×AGE interaction: F(8,412)=8.91, p<0.001, ηp2=0.15). We found that the participants from 9 years of age showed similar extent of sequence learning as the adult groups to 44 years of age (all p’s>0.25), but the learning in 4–8 years of age was smaller compared to these adult groups (all p’s<0.014). At older ages, there was a decline in the sequence learning, with both the 45–59 and 60–85-year-old group differing significantly from the groups between the 11–44 years of age (all p’s<0.025).
Figure 2.
Sequence learning measured the z-transformed RT data in all groups.
The analysis of the response accuracy further enhanced the picture emerging from the results with RTs. We found a significantly greater accuracy for high- than low-frequency triplets (main effect of TRIPLET TYPE: F(1,412)=217.14, p<0.001, ηp2=0.345). Although all age groups older than 6 showed significant sequence learning (all p’s<0.011), the age groups differed significantly in the strength of the sequence learning (TRIPLET TYPE × AGE interaction: F(8,412)=3.73, p<0.001, ηp2=0.07). Whereas groups between 7 and 44 years of age showed similar degrees of learning, this was significantly higher than the youngest (4–6) and the two oldest (45–59 and 60–85) groups (p’s<0.03) (Figure 1d).
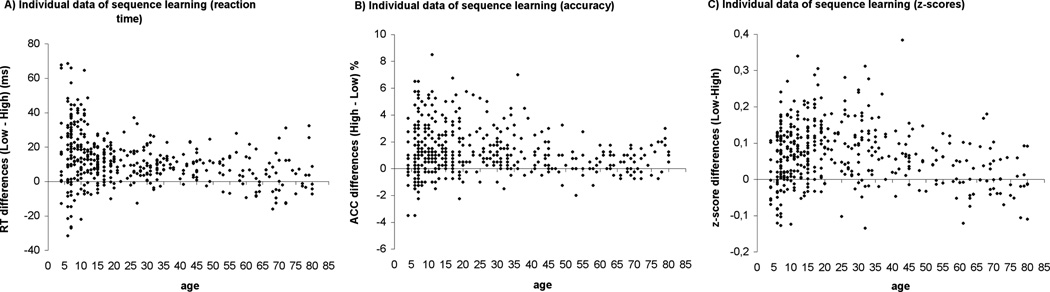
Figure 3 shows the individual data for sequence learning measured by raw RT, accuracy and z-transformed RT data. The pattern of this data is consistent with the ANOVA results: 1) children between 4–12 years of age showed greater sequence learning as measured by raw RT, whereas 2) in sequence-specific learning as measured by accuracy and z-transformed RTs adults exhibited the highest performance.
Figure 3.
Individual data for sequence learning measured by raw RT (A),accuracy (B), and z-scores (C) in all ages.
Discussion
The goal of the present study was to investigate the differences in implicit skill learning across the human life span. This work extends previous studies (Bennett et al., 2007; Gaillard et al., 2009; J. H. Howard, Jr. & Howard, 1997; Meulemans et al., 1998; Thomas et al., 2004) in two ways: 1) it examined a wide range of ages between 4–85 years, and 2) it used a probabilistic task, which enabled us to measure the “pure” sequence-specific learning defined by the sensitivity to raw probabilities of high and low frequency events. We found that the 4- to 12-year-old age groups showed the strongest learning effect measured by the raw RT difference scores. Around the age of 12, we found a striking transition to less pronounced sequence-specific learning, as measured by smaller differences between the responses to high and low frequency triplets. Confirming earlier results (D. V. Howard et al., 2004; J. H. Howard, Jr. & Howard, 1997; Nemeth, Janacsek, Londe et al., 2010), we found that this learning capacity was significantly reduced in the oldest age group. Thus, in contrast to the developmental invariance (Reber, 1993) and the age-related changes approaches (Meulemans et al., 1998; Vinter & Perruchet, 2000), our results demonstrate a gradual decline in learning across the lifespan.
Sequence learning scores based on the accuracy and raw reaction time showed different curves: the former one is a bell-shaped curve, whereas the latter is a gradually declining curve (Figure 1c–d). Hence, these two types of learning scores can reflect different underlying mechanisms and brain systems. The accuracy learning score may be more related to attention, mainly voluntary attention, whereas the RT learning score may be related to involuntary attention and intuitive processes (Burgess, Gilbert, & Dumontheil, 2007; Prinzmetal, McCool, & Park, 2005). The relatively weaker accuracy learning effects in children and older groups may be due to the underdeveloped/deteriorating attentional brain circuits connected to the frontal lobe.
Our study raised a methodological issue which affects the developmental studies in general. It is a long-standing issue in the literature how to compare groups with different baseline speeds. It could be argued that the youngest groups in our study have larger raw RT learning scores (more difference between high and low frequency triplets) because they have more room to do so, given their longer RTs. However this argument does not seem to hold in our study, because the oldest group showed equally long RTs as 9–10 year-olds, yet they had a four-fold reduced learning score (Figure 1). Along a similar line of argument, the accuracy measures of the youngest group (4–6 year-olds) and the oldest groups (45–85 year-olds) showed a marked difference (~8%) in general accuracy, giving more room for the young group to produce larger differences between high and low frequency triplets, yet the actual sequence-learning (i.e. high-low differences in accuracy) showed no difference. In contrast, the two smallest age groups had almost identical general accuracy yet there was a more than two-fold increase in sequence learning in the second youngest group (7–8 year-olds). Thus, our data suggests no linear relationship between the general magnitude of reaction time/accuracy and the learning measures.
A second customary approach to the problem of comparing groups with different baseline speeds is to analyze the data using z-transformation. Z-scores of our results show a different picture than raw the RT data analysis: the learning performances from 9 to 44 years of age are similar with weaker performance in the younger and older age groups. The z-transformation is often used to control general processing speed across different age groups in developmental studies. However, the main function of z-transformation is not to control the processing speed, but to normalize the distribution of responses. Thus, z-transformation has mathematical assumptions about the form of distributions and is therefore not theory independent (Yap, Balota, Sibley, & Ratcliff, in press). Z scoring fully adjusts for processing speed only if all participants have the same type of distributions. Therefore using z-scores in developmental studies might be misleading. Furthermore, it is unclear how general processing speed and variability contributes to learning and performance in different ages. In recent years, several studies analyzed the variability and noise across a wide age-range (Der & Deary, 2006; Hultsch, MacDonald, Hunter, Levy-Bencheton, & Strauss, 2000; Rabbitt, Osman, Moore, & Stollery, 2001). For example, Rabbitt et al. (2001) found that people’s fastest RT’s were relatively unaffected by age, but the number of unnecessarily slow responses was higher in older ages, and, thus, the increase in the mean RT was a result of increasing variability, which was an important component of cognitive aging. Moreover, several studies outline that the noise and the performance variability enables adaptive plasticity of motor skills (Slifkin & Newell, 1998, 1999; Turner & Brainard, 2007) and high variability can support effective learning and performance (Sanger, 2010). Thus, based on previous studies and on our analyses, we suggest that both the processing speed (mean reaction time) and variability are inherent aspects of development and aging. We think that the z-transformation eliminates these inherent aspects of learning, making the transformed results difficult to understand and explain.
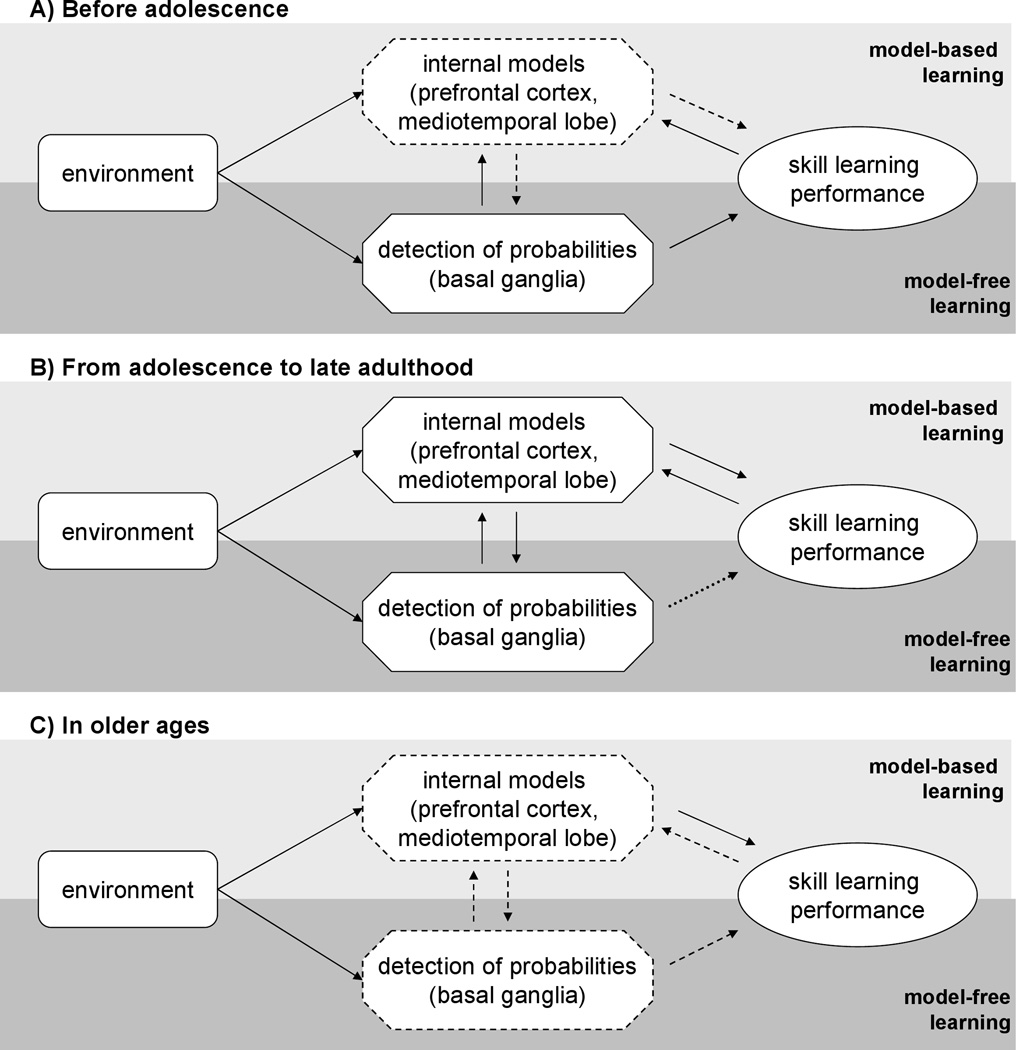
Based on the reasoning above, we propose that, the raw RT difference between the high and low frequency triplets in the ASRT task is a measure of human sensitivity to the relative raw probabilities of events observed implicitly in their environment. Thus, our results show a marked decrease in this sensitivity around the age of 12, which is in contrast to the traditional view of a steady improvement of cognitive learning abilities until late in adulthood (Craik & Bialystok, 2006). However, this discrepancy might be explained based on a shift in the structural development of implicit learning. Although the raw probabilities of the sensory environment are important for learning and both infants (Aslin, Saffran, & Newport, 1998; Fiser & Aslin, 2002; Saffran et al., 1996; Saffran, Johnson, Aslin, & Newport, 1999) and adults (Fiser & Aslin, 2001; Hunt & Aslin, 2001) are highly sensitive to them, there is an ongoing debate on how using these simple probabilities can lead to highly complex knowledge of the world, such as sensory invariances and development of a language (Gomez & Gerken, 1999; Marcus, Vijayan, Rao, & Vishton, 1999). Recent studies proposed that using an internally stored structured model of the world that emerges based on past experience together with probabilistic learning could help to address this issue and also provide evidence that humans might implement such a strategy during implicit learning (Orban, Fiser, Aslin, & Lengyel, 2008; Tenenbaum, Kemp, Griffiths, & Goodman, 2011). In this framework, as the internal model develops with experiences becoming more influential, internal interpretations of events become more elaborate and less directly related to their raw probabilities. A recent study argued that from a normative standpoint, existence of multiple learning mechanisms in the brain (cf. model-free vs. model-based learning) with an uncertainty–based arbitration between them would be computationally optimal (Daw et al., 2005). Anchoring this hypothesis biologically, the presumed mechanisms related to these two types of learning were suggested to be related to the prefrontal areas and temporal lobe of the cortex, respectively (Daw et al., 2005). Support for the separated and complementary nature of the prefrontal- and medial temporal lobe (MTL)-dependent learning based on internal models vs. basal ganglia-dependent model-free learning comes from various studies investigating learning under specific conditions. These studies showed that obstructing the PFC and/or MTL by a demanding secondary task (Foerde, Knowlton, & Poldrack, 2006) do not adversely affect implicit learning. Other studies found that inserting a task between the learning sessions (Brown & Robertson, 2007a, 2007b), performing a working memory and an implicit learning task simultaneously (Filoteo, Lauritzen, & Maddox, 2010), or a neuropharmacological blockage (Frank, O'Reilly, & Curran, 2006) even had a positive effect on performance in implicit learning task. Importantly, it is known that the cortical areas connected to the internal models related to model-based learning become truly functional late in the development, around age of 12 (Blakemore & Choudhury, 2006; Giedd, et al., 1999), which is about the age when we found the sudden decrement in sensitivity to the relative raw probabilities. We propose, that this enhanced functionality signals the shift when the system adapts efficiently to more complex aspects of the world by relying more on internal model-based interpretations, while somewhat neglecting the raw probabilities of the sensory input (Figure 4a–b), and therefore, decreasing the ability to develop and stabilize fundamentally new basic competences. Thus the seemingly paradoxical result of gradually becoming less sensitive to basic statistics, if timed appropriately, could be the optimal strategy for human implicit learning in general.
Figure 4.
Competition between model-based and model-free neurocognitive subsystems of skill learning across lifespan. (A) Before adolescence, underdeveloped internal models (dashed boundary) have little influence on interpretations of detected raw statistical probabilities of events in the environment (dashed arrows). Skill learning performance is determined by raw probabilities. (B) From adolescence to late adulthood, well-developed internal models (solid boundary) strongly modulate the interpretations of observed statistics of the input. This helps extracting complex relations but relatively impairs measuring and utilizing raw probabilities in skill learning (dotted arrow). (C) In older ages, skill learning performance decreases. This decline could be caused by the combination of reduced sensitivity to raw statistical probabilities (dashed boundary), increasingly rigid internal models (dashed boundary) and/or weaker connection between these systems (dashed arrows).
Our results did not reveal any differences between the young adults and middle-age groups. Salthouse’s (1996) “simultaneity mechanism” theory of cognitive aging predicts the age-related deficits in probabilistic sequence learning (Curran, 1997; J. H. Howard, Jr. & Howard, 1997). Feeney, Howard & Howard (2002) found age-related deficits in pattern sensitivity in “older” (mean age: 49.4) compared with “younger” (mean age: 41.4) middle-aged groups. These different results could be related to that Feeney used a smaller sample size, a longer version of the ASRT and different method of analysis.
What are the underlying mechanisms of the decreased performance of the elderly group? In a recent fMRI study, Dennis and Cabeza (in press) showed that older adults recruited the MTL for implicit learning, and this activation was significantly greater, while striatal activity decreased in older people compared with young adults during implicit learning. In a recent study, Rieckmann, Fisher & Backman (2010) found similar results: in young adults during the learning session, the activation of the striatum increased, but the that of MTL decreased. By contrast, in older adults, sequence learning positively related to activation increases in both the striatum and MTL. Using multimodal imaging measures, Giorgo et al. (2010) found extensive reductions in the gray matter volume in aging, but reductions were detected earlier in the frontal cortex. Furthermore, a recent diffusion tensor imaging aging study by Bennett et al. (in press) found that the caudate–dorsolateral prefrontal cortex (DLPFC) and hippocampus-DLPFC tract integrity were related to ASRT sequence learning. The caudate-DLPFC tract integrity decreased in the older ages, mediating age-related differences in sequence learning. Within the computational framework proposed by Daw and collegues (2005), these findings can be interpreted as a deterioration in three mechanisms that contribute to the age-related decline in skill learning: 1) reduced detection of probabilities, 2) rigidity of internal models and/or 3) more restricted connections between internal models and probability detection (Figure 4c). Thus, not only the model-free, but also the model-based learning, might be limited in older ages. Future studies are needed to systematically examine the underlying neural mechanisms of age-related differences in skill learning.
In summary, based on our raw RT results we suggest that acquiring fundamentally new skills that cannot be derived from skills already possessed is the most effective before adolescence, and it might be largely based on the fronto-striatal circuitry, such as the basal ganglia and cerebellum, in agreement with earlier skill learning models (Doyon et al., 2009; Hikosaka et al., 1999; Hikosaka et al., 2002) and computational learning models (Daw et al., 2005). Our findings are in good agreement with everyday life experience showing that an early (~ before 12 years) start of learning some sports, music instruments, second language, etc. often leads to higher level of competence. These results may have implications for the development of learning and memory, facilitating new skill training and pedagogic methods (e.g., for teaching languages) and may also contribute to the understanding of neurodevelopmental and age-related disorders (e.g., autism, SLI, dyslexia and dementia) and lead to relevant treatment options.
Acknowledgement
Thanks to Darlene V. Howard, James H. Howard, Jr. and Michael Ullman for numerous illuminating discussions. Thanks also to Robert Mingesz, Attila Krajcsi, István Winkler, Gábor Orosz, Ágnes Lukács, Ágnes Kovács and Zsuzsa Londe for their comments on the previous version of the manuscript. This research was supported by the Bolyai Scholarship Program (to D.N.), by OTKA K 82068 (to D. N.), and by NIH NEI R01 EY018196 (to J.F.).
Footnotes
The authors report no conflict of interest and have no financial disclosure.
References
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58(2):261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Saffran JR, Newport EL. Computation of conditional probability statistics by 8-month old infants. Psychological Science. 1998;9(4):321–324. [Google Scholar]
- Barnes KA, Howard JH, Jr, Howard DV, Gilotty L, Kenworthy L, Gaillard WD, et al. Intact implicit learning of spatial context and temporal sequences in childhood autism spectrum disorder. Neuropsychology. 2008;22(5):563–570. doi: 10.1037/0894-4105.22.5.563. [DOI] [PubMed] [Google Scholar]
- Barnes KA, Howard JH, Jr, Howard DV, Kenealy L, Vaidya CJ. Two forms of implicit learning in childhood ADHD. Developmental Neuropsychology. 2010;35(5):494–505. doi: 10.1080/87565641.2010.494750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Howard JH, Jr, Howard DV. Age-Related Differences in Implicit Learning of Subtle Third-Order Sequential Structure. Journal of Gerontology: Psychological Sciences. 2007;62B(2):98–103. doi: 10.1093/geronb/62.2.p98. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden D, Vaidya C, Howard J, Howard D. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.03.017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(3–4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nature Neuroscience. 2007a;10(2):148–149. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Off-line processing: Reciprocal interactions between declarative and procedural memories. Journal of Neuroscience. 2007b;27(39):10468–10475. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, White DA, Mandernach T, Keys BA. Inhibitory control across the life span. Developmental Neuropsychology. 2001;20(3):653–669. doi: 10.1207/S15326942DN2003_7. [DOI] [PubMed] [Google Scholar]
- Clohessy AB, Posner MI, Rothbart MK. Development of the functional visual field. Acta Psychologica. 2001;106(1):51–68. doi: 10.1016/s0001-6918(00)00026-3. [DOI] [PubMed] [Google Scholar]
- Cohen D, Pascual-Leone A, Press D, Robertson E. Off-line learning of motor skill memory: A double dissociation of goal and movement. Proceedings of the National Academy of Sciences. 2005;102(50):18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. TRENDS in Cognitive Sciences. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of aging on implicit sequence learning: accounting for sequence structure and explicit knowledge. Psychological Research. 1997;60(1–2):24–41. doi: 10.1007/BF00419678. [DOI] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Dennis N, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.04.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der G, Deary IJ. Age and sex differences in reaction time in adulthood: Results from the United Kingdom Health and Lifestyle Survey. Psychology and Aging. 2006;21(1):62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behavioral Brain Research. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Feeney JJ, Howard JH, Howard DV. Implicit learning of higher order sequences in middle age. Psychology and Aging. 2002;17(2):351–355. [PubMed] [Google Scholar]
- Filoteo JV, Lauritzen S, Maddox WT. Removing the Frontal Lobes. Psychological Science. 2010;21(3):415–423. doi: 10.1177/0956797610362646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser J, Aslin RN. Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychological Science. 2001;12(6):499–504. doi: 10.1111/1467-9280.00392. [DOI] [PubMed] [Google Scholar]
- Fiser J, Aslin RN. Statistical learning of new visual feature combinations by infants. Proceedings of the National Academy of Sciences. 2002;99(24):15822–15826. doi: 10.1073/pnas.232472899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J, Maybery MT, Bennett S. Implicit learning differences: A question of developmental level? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(1):246–252. doi: 10.1037//0278-7393.26.1.246. [DOI] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proceedings of the National Academy of Science. 2006;103(31):11778–11783. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, O'Reilly RC, Curran T. When Memory Fails, Intuition Reigns: Midazolam Enhances Implicit Inference in Humans. Psychological Science. 2006;17(8):700–707. doi: 10.1111/j.1467-9280.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Frensch PA, Miner CS. Effects of presentation rate and individual differences in short-term memory capacity on an indirect measure of serial learning. Memory and Cognition. 1994;22(1):95–110. doi: 10.3758/bf03202765. [DOI] [PubMed] [Google Scholar]
- Gaillard V, Destrebecqz A, Michiels S, Cleeremans A. Effects of age and practice in sequence learning: A graded account of ageing, learning, and control. European Journal of Cognitive Psychology. 2009;21(2):255–282. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RL, Gerken L. Artificial grammar learning by 1-year-olds leads to specific and abstract knowledge. Cognition. 1999;70(2):109–135. doi: 10.1016/s0010-0277(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, et al. Parallel neural networks for learning sequential procedures. Trends in Neurosciences. 1999;22(10):464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Current Opinion in Neurobiology. 2002;12(2):217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH. Adult age differences in the rate of learning serial patterns: Evidence from direct and indirect tests. Psychology and Aging. 1992;7(2):232–241. doi: 10.1037//0882-7974.7.2.232. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH., Jr Age differences in learning serial patterns: direct versus indirect measures. Psychology and Aging. 1989;4(3):357–364. doi: 10.1037//0882-7974.4.3.357. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Jr, Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychology and Aging. 2004;19(1):79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV. Age differences in implicit learning of higher-order dependencies in serial patterns. Psychology and Aging. 1997;12(4):634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, Hunter MA, Levy-Bencheton J, Strauss E. Intraindividual variability in cognitive performance in older adults: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000;14(4):588–598. doi: 10.1037//0894-4105.14.4.588. [DOI] [PubMed] [Google Scholar]
- Hunt RH, Aslin RN. Statistical learning in a serial reaction time task: access to separable statistical cues by individual learners. Journal of Experimental Psychology: General. 2001;130(4):658–680. doi: 10.1037//0096-3445.130.4.658. [DOI] [PubMed] [Google Scholar]
- Keele SW, Ivry R, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychological Review. 2003;110(2):316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Kirkham N, Slemmer J, Richardson D, Johnson S. Location, location, location: Development of spatiotemporal sequence learning in infancy. Child Development. 2007;78(5):1559–1571. doi: 10.1111/j.1467-8624.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Intuition: a social cognitive neuroscience approach. Psychological Bulletin. 2000;126(1):109–137. doi: 10.1037/0033-2909.126.1.109. [DOI] [PubMed] [Google Scholar]
- Marcus GF, Vijayan S, Rao SB, Vishton PM. Rule Learning by seven-month-old infants. Science. 1999;283:77–80. doi: 10.1126/science.283.5398.77. [DOI] [PubMed] [Google Scholar]
- Maybery M, Taylor M, O'Brien-Malone A. Implicit learning: Sensitive to age but not IQ. Australian Journal of Psychology. 1995;47(1):8–17. [Google Scholar]
- Meulemans T, Van der Linden M, Perruchet P. Implicit sequence learning in children. Journal of Experimental Child Psychology. 1998;69(3):199–221. doi: 10.1006/jecp.1998.2442. [DOI] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K, Balogh V, Londe Z, Mingesz R, Fazekas M, et al. Learning in Autism: Implicitly Superb. PLoS ONE. 2010;5(7):e11731. doi: 10.1371/journal.pone.0011731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K, Londe Z, Ullman MT, Howard D, Howard J. Sleep has no critical role in implicit motor sequence learning in young and old adults. Experimental Brain Research. 2010;201(2):351–358. doi: 10.1007/s00221-009-2024-x. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Orban G, Fiser J, Aslin RN, Lengyel M. Bayesian learning of visual chunks by human observers. Proceedings of the National Academy of Sciences. 2008;105(7):2745–2750. doi: 10.1073/pnas.0708424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchet P, Pacton S. Implicit learning and statistical learning: One phenomenon, two approaches. Trends in Cognitive Sciences. 2006;10(5):233–238. doi: 10.1016/j.tics.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, et al. The Neural Correlates of Motor Skill Automaticity. Journal of Neuroscience. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzmetal W, McCool C, Park S. Attention: Reaction time and accuracy reveal different mechanisms. Journal of Experimental Psychology: General. 2005;134(1):73–92. doi: 10.1037/0096-3445.134.1.73. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Osman P, Moore B, Stollery B. There are stable individual differences in performance variability, both from moment to moment and from day to day. The Quarterly Journal of Experimental Psychology Section A. 2001;54(4):981–1003. doi: 10.1080/713756013. [DOI] [PubMed] [Google Scholar]
- Reber AR. Implicit learning and tacit knowledge: An essay on the cognitive unconscious. Vol. 19. New York: Oxford University Press; 1993. [Google Scholar]
- Remillard G. Implicit learning of second-, third-, and fourth-order adjacent and nonadjacent sequential dependencies. The Quarterly Journal of Experimental Psychology. 2008;61(3):400–424. doi: 10.1080/17470210701210999. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, Bäckman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: Relations to performance. Neuroimage. 2010;50(3):1303–1312. doi: 10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Johnson EK, Aslin RN, Newport EL. Statistical learning of tone sequences by human infants and adults. Cognition. 1999;70(1):27–52. doi: 10.1016/s0010-0277(98)00075-4. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sanger TD. Controlling variability. Journal of Motor Behavior. 2010;42(6):401–407. doi: 10.1080/00222895.2010.526496. [DOI] [PubMed] [Google Scholar]
- Schendan H, Searl M, Melrose R, Stern C. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37(6):1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Slifkin AB, Newell KM. Is variability in human performance a reflection of system noise? Current Directions in Psychological Science. 1998;7(6):170–177. [Google Scholar]
- Slifkin AB, Newell KM. Noise, information transmission, and force variability. Journal of Experimental Psychology: Human Perception and Performance. 1999;25(3):837. doi: 10.1037//0096-1523.25.3.837. [DOI] [PubMed] [Google Scholar]
- Soetens E, Melis A, Notebaert W. Sequence learning and sequential effects. Psychological Research. 2004;69(1):124–137. doi: 10.1007/s00426-003-0163-4. [DOI] [PubMed] [Google Scholar]
- Song S, Howard JH, Jr, Howard DV. Sleep does not benefit probabilistic motor sequence learning. Journal of Neuroscience. 2007;27(46):12475–12483. doi: 10.1523/JNEUROSCI.2062-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences USA. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum JB, Kemp C, Griffiths TL, Goodman ND. How to grow a mind: Statistics, structure, and abstraction. Science. 2011;331(6022):1279–1285. doi: 10.1126/science.1192788. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Hunt RH, Vizueta N, Sommer T, Durston S, Yang Y, et al. Evidence of Developmental Differences in Implicit Sequence Learning: An fMRI Study of Children and Adults. Journal of Cognitive Neuroscience. 2004;16(8):1339–1351. doi: 10.1162/0898929042304688. [DOI] [PubMed] [Google Scholar]
- Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford: Oxford University Press; 2000. [Google Scholar]
- Turner EC, Brainard MS. Performance variability enables adaptive plasticity of 'crystallized' adult birdsong. Nature. 2007;450(7173):1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Vinter A, Perruchet P. Implicit learning in children is not related to age: evidence from drawing behavior. Child Development. 2000;71(5):1223–1240. doi: 10.1111/1467-8624.00225. [DOI] [PubMed] [Google Scholar]
- Yap MJ, Balota DA, Sibley DE, Ratcliff R. Individual Differences in Visual Word Recognition: Insights From the English Lexicon Project. Journal of experimental psychology. Human perception and performance. doi: 10.1037/a0024177. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]