Abstract
The development of T cell self-tolerance in the thymus is important for establishing immune homeostasis and preventing autoimmunity. Here, we review the components of T cell tolerance, which includes TCR self-reactivity, costimulation, cytokines, and antigen presentation by a variety of APCs subsets. We discuss the current evidence on the process of regulatory T (Treg) cell and negative selection and the importance of TCR signaling. We then examine recent evidence showing unique roles for bone marrow (BM)-derived APCs and medullary thymic epithelial cells (mTECs) on the conventional and Treg TCR repertoire, as well as emerging data on the role of B cells in tolerance. Finally, we review the accumulating data that suggest that cooperative antigen presentation is a prominent component of T cell tolerance. With the development of tools to interrogate the function of individual APC subsets in the medulla, we have gained greater understanding of the complex cellular and molecular events that determine T cell tolerance.
Keywords: Dendritic Cells, T Cells, Tolerance/Suppression/Anergy, Thymus, Medullary Thymic Epithelial Cells, Antigen Presentation/Processing
Introduction
A defining feature of adaptive immunity is its ability to harness somatic gene rearrangement to generate a diverse antigen receptor repertoire, which allows for the recognition of a variety of pathogens including those not previously encountered by the host species. For most T cells, rearrangement of the TCR α and β chains occurs early during T cell development in a specialized organ–the thymus. Thymocytes expressing functional TCR genes can then undergo positive selection, where weak TCR recognition of self-antigen MHC complexes on cortical thymic epithelial cells allows continued T cell development and CD4 and CD8 lineage commitment (1–3). These mechanisms serve to generate the T cell population responsible for immunity to pathogens.
However, the potential cost of this randomly generated TCR repertoire is the presence of TCRs that recognize self-antigens and contribute to the pathogenesis of autoimmune disease. An important component of T cell development is therefore to limit the autoreactivity within the T cell population in the thymus prior to their release into the periphery of the host. This process in the thymus, generally referred to as central tolerance, is achieved through deletion of autoreactive clones (i.e. negative selection), and for CD4+ T cells, diversion of autoreactive clones into the regulatory T (Treg) cell lineage (4–6), a subset of T cells that inhibit, rather than induce, inflammatory responses (7).
Although central tolerance can occur throughout the thymus (2), the majority of this process appears to occur in the medulla, which consists of a secondary lymphoid tissue-like structure populated by hematopoiesis-derived dendritic cells, macrophages, and B cells; as well as thymic epithelial cells (mTECs) and fibroblasts (8, 9). Thus, a diverse assortment of cell types appears to be involved in central tolerance.
In this review, we will discuss recent advances in understanding how T cells develop tolerance to self in the thymus. We will first focus on the signals that trigger the process of negative or Treg cell selection in developing thymocytes, including those from cytokine, costimulation, and T cell receptors. We will then examine how the various medullary antigen presenting cells (APCs) shape the post-thymic TCR repertoire. Finally, we will conclude by discussing the transcription factor Autoimmune regulator (Aire) and its role in coordinating collaboration between mTECs and DCs to facilitate T cell tolerance.
Signaling T cells to become tolerant
It has been evident for some time that a certain level of self-reactivity drives the deletion of thymocytes from the T cell repertoire (reviewed in (10)). A majority of evidence also suggests that self-reactivity drives CD4+ thymocytes into the Treg cell lineage. Although this issue is still debated, recent studies have provided additional details regarding the range of self-reactivity that facilitates Treg cell versus negative selection, as well as the role of other factors such as IL-2 in determining Treg cell fate.
TCR signal strength and intraclonal competition for T cell tolerance induction
A substantial body of work over the past 15 years have supported the notion that TCR self-reactivity is an important factor in thymic Treg cell selection based on several lines of evidence (summarized in (4)). First, studies of TCR and antigen transgenic mice showed that cognate antigen recognition in the thymus was required for Treg cell selection (11–15). Second, TCR repertoire analyses demonstrated marked differences between Foxp3+ and Foxp3− cells, consistent with an important role for TCR specificity in Treg cell selection (16, 17). More recently, the use of surrogate markers of TCR activation such as Nur77 (18), and the development of TCR transgenic mice expressing thymic Treg TCRs (12, 19, 20) have provided additional supporting evidence that Treg cell selection is driven by TCR specificity (Figure 1).
Figure 1.

One interesting observation from studies of natural Treg TCR transgenic cells is that Treg cell development is typically limited by intraclonal competition, such that low clonal frequencies are required to observe appreciable frequencies of Foxp3+ cells in the thymus (12, 19, 20). This suggests that the ligands for these natural Treg TCRs are not abundant and perhaps presented by small numbers of APCs, resulting in a limited “niche” for Treg cell selection. Note that these data do not imply that this type of “niche” is required for Treg cell selection, nor does it address the role of other factors such as cytokines, that may affect Treg cell numbers as different kind of “niche.” In addition to the notion that natural thymic Treg cell selection is typically associated with intraclonal competition for antigen, these data showed that individual Treg TCRs vary considerably in their efficiency for inducing Foxp3. However, quantification of the degree of self-reactivity that initiated Treg cell development versus negative selection could not be inferred from those studies as the self-ligands were unknown.
To address this question, we generated a panel of TCRs with a large range of sensitivity to ovalbumin (OVA) to test Treg cell selection in vivo (21). Analysis of this panel of TCRs revealed that in vivo encounter with OVA expressed via a rat-insulin-promoter (RIP-mOVA) transgene induced Treg cell selection with an efficiency directly proportional to the in vitro sensitivity of the TCR to OVA. The range of sensitivity to OVA that could elicit any detectable Treg cell selection was broad (1000-fold) compared to affinity ranges required for positive selection of conventional T (Tconv) cells or negative selection. Detectable negative selection occurred only with sensitivity that was 100-fold higher than that required for Treg cell selection (21). Thus, these data support the notion that thymic Treg cell selection is driven by TCR specificity, and show that the efficiency of this process is directly correlated to the degree of self-reactivity in a large continuum with negative selection.
Recently, crystal structures of two human induced Treg TCRs bound to their cognate antigens were reported showing a 180 degree reversed polarity in their interaction with peptide/MHC compared with all other TCR:peptide/MHC structures (22). Whether this is a general feature of naturally occurring thymic Treg TCRs remains to be determined. However, it is clear that “standard” TCR interactions with peptide/MHC are capable of inducing Treg cell selection in murine TCR transgenic models. Similarly, the OVA-reactive TCRs described above all utilized the same TCRα family paired with the same TCRβ chain from the OVA-reactive DO11 TCR suggesting that recognition of OVA over a broad range occurs in the same TCR:peptide/MHC configuration. Nonetheless, this new report brings up the intriguing possibility that the configuration of TCR recognition of peptide/MHC, via an unknown mechanism independent of affinity, may affect Treg cell selection.
Others, however, have suggested that self-reactivity is not responsible for specifying thymic Treg cell generation. For example, a recent study using mice engineered to express a single dominant peptide:MHC argued against the notion that high affinity self-reactive TCRs lead to Treg cell commitment (23). Whether the constraint of limiting the peptide repertoire to a single epitope explains the differing interpretations is unclear. Similarly, it has been reported that there is considerable overlap in the affinity for Myelin Oligodendrocyte Glycoprotein (MOG) between Treg and Tconv cells during experimental autoimmune encephalomyelitis (EAE) using 2D affinity measurements (24), which is consistent with previous TCR repertoire studies (25, 26). These studies suggest that reactivity to MOG was not strongly involved in thymic Treg cell selection, arguing against self-recognition as a driving factor in Treg cell selection. However, it may be possible that higher affinity MOG specific cells are preferentially found in the Treg cell subset, but that the immunization protocol changes this balance by expanding higher affinity Tconv cells. Alternatively, MOG may be poorly presented in the thymus, such that the thymically derived MOG-reactive Treg cells are actually selected on other self-antigens. Reactivity of Treg cells to antigens not present in the thymus has been described, as we found several OVA-reactive TCRs that undergo thymic Treg cell selection in the absence of RIP-mOVA (21). Identification of natural Treg cell epitopes will be required to definitively address the role of self-reactivity in thymic Treg cell selection.
Role of IL-2 in Foxp3 induction versus Treg cell survival
While TCR interaction with antigen may be considered the principle signal in Treg cell selection, cytokines and costimulation also play important roles. It has been proposed that TCR stimulation in Foxp3− CD4+ thymocytes induces CD25, the alpha-chain of the high affinity IL-2 receptor. In turn, IL-2, and to a lesser extent IL-15, can then directly induce Foxp3 expression (Figure 1, (27–29)). In addition to IL-2, the cytokine TGFβ is reported to be involved in Treg cell selection (30) or survival (31). In adult mice, IL-2 may be more important for Treg development as TGFβ-deficiency does not markedly alter the number of Treg cells beyond the neonatal period (32).
Previous studies showed that CD25-deficiency does not completely abrogate induction of Foxp3. Rather, the frequency of Treg cells was reduced by 80% in CD25-deficient cells versus CD25-sufficient cells (33). This was in part attributed to the partially redundant effect of IL-15 in place of IL-2 (34, 35). Recently, it was proposed that Foxp3 induction prior to CD25-upregulation in response to TCR signals may be the major contributor to the mature Treg cell population (Figure 1, (36)). They observed that expression of Foxp3 is inherently pro-apoptotic, requiring rescue from death by IL-2 signaling and subsequent upregulation of Bcl-2 (36). In this model, most developing Treg cells die because of limiting IL-2 in the thymus. However, it remains unclear whether negative selection in CD4+ T cells in general occurs via a Foxp3-dependent pathway.
Future work will be required to determine the factors that result in the initial induction of CD25 rather than Foxp3. Possibilities include the extent of CD28 costimulation (discussed below), the strength of TCR signals, the type of APC, or the number of serial contacts of T cells with different antigen-bearing APCs. Another question will be to determine the relative contribution between the CD25 versus Foxp3 first pathways to mature Treg cells, perhaps via TCR repertoire analysis of Foxp3lowCD25− versus Foxp3− CD25+ cells. Although these two pathways differ in the mechanism by which IL-2 is involved (Figure 1), the pathways are functionally convergent in that both involve IL-2 signals for establishment of the mature Treg cell population.
Dendritic cell production of IL-2
T cells were proposed to be the source of IL-2 for Treg cell generation (37). However, a recent paper using an in vitro thymic slice model suggested that dendritic cells may the primary source of IL-2 and IL-15 (38). In this model, they observed that the frequency of Treg cell development increased proportional to antigen availability, consistent with the notion that TCR signal strength is important in Treg cell selection (21). However, increased precursor frequency did not decrease the efficiency of Treg cell development, contrary to studies with natural Treg TCRs described above showing intraclonal competition. This may be due to the use of exogenously added antigen to the thymic slice, leading to diffuse antigen presentation by thymic APCs and a non-limiting antigenic “niche.” Competition for IL-2 also occurs with pre-existing Treg cells resulting in restriction of overall Treg cell numbers, a phenomenon also observed in the periphery (39).
A second report suggested that Treg cells recirculating from the periphery can also limit thymic Treg cell development by competing for IL-2 (40). This finding is consistent with another paper using the Rag-GFP reporter (18) showing that many thymic Foxp3+ cells in the thymus are older than conventional thymocytes, suggesting that they are recirculated from the periphery or have been resident in the thymus for a longer period of time than conventional T cells. Interestingly, recirculation of Treg cells from the periphery could explain the presence of Treg cells in the thymus with specificity for microbial antigen (41), in addition to the possibility that these foreign antigen-reactive TCRs cross-react with self-antigen (21). However, a separate study utilizing an alternative lineage tracing strategy suggested that Treg cell retention also constitutes part of the pool of Rag-GFP− cells and account for the majority of thymic Treg cells in older mice (42). Thus, while thymic recirculation of Treg cells from the periphery has been clearly established, the relative contribution of thymic Treg cells from intrathymic development versus peripheral recirculation requires further clarification.
The factors contributing to retention or recirculation of thymic Treg cells remain unknown. In addition, the immunologic benefit of a negative feedback loop for thymic Treg cell development is unclear. If IL-2 rescues Foxp3+ cells from death, then limiting IL-2 in the thymus would lead to enhanced negative selection. However, if low IL-2 decreases Treg cell selection, this may facilitate the release of self-reactive conventional T cells, perhaps promoting autoimmunity with age.
Important role for costimulation in thymic Treg cell generation
The interaction of TCR with self-peptide:MHC alone does not efficiently drive Treg cell development. It was discovered a number of years ago that costimulation by CD28 was important for Treg cell development (43, 44). Subsequent studies have suggested that CD28 is important for generating an IL-2 responsive CD25+ Foxp3− CD4SP precursor (28, 45). CD28 is also thought to be important for driving the development of Foxp3low CD25− cells in the Treg cell maturation process (37).
Interestingly, the loss of CD28-dependent signals do not appear to markedly affect the Treg TCR repertoire even though there is a 3–5 fold reduction in the absolute number of thymic Treg cells generated (28, 45). This suggests that CD28 signals primarily affect Treg cell survival. By contrast, IL-2 can affect the TCR repertoire of Treg cells, as assessed by the use of a hypersensitive mutant of Stat5, a factor involved in IL-2 signals (29). Thus, even though CD28 and IL-2 signals may be associated with TCR activation, IL-2 has a greater impact on determine whether T cells become Foxp3+, either via induction of Foxp3 or survival of Foxp3+ cells.
In addition to CD28, other costimulatory factors have been reported to be involved in increasing the number of Treg cells generated in the thymus. These include members of the tumor-necrosis factor (TNF) receptor superfamily (TNFRSF, GITR, OX40 and TNFR2) (46), Id1(47), CD40-CD40L interactions (48–50), and CD27-CD70 co-stimulation (51). The impact of these costimulation signals on the Treg TCR repertoire is unclear.
While recent studies have shed light on the process of thymic Treg cell selection, several important questions remain. First, identification of endogenous Treg cell ligands and their corresponding TCRs would be useful to determine the affinity range of Treg cell selection. This would also enable dynamic study of thymocyte:APC interactions with two-photon microscopy, as well as assessment of the APC type and their distribution throughout the medulla. Second, the molecular events that control Foxp3 induction remain imprecisely defined as studies often report total Treg cell numbers or frequencies. Analysis of the TCR repertoire would be useful to determine whether these molecules affect TCR-dependent Treg cell selection (e.g. adding or subtracting TCRs from the repertoire) versus promote the survival of Foxp3+ cells–two non-mutually exclusive possibilities. Finally, understanding the mechanism that results in the initial upregulation of CD25 versus Foxp3 may be instructive in understanding how the Foxp3 locus is opened and transcribed, as it appears that there may be multiple roads to get to Foxp3.
Role of mTECs and BM APCs in self-tolerance
The importance of TCR specificity in the process of T cell tolerance necessitates an effective means of thymic presentation of the self-antigenic repertoire, including those antigens expressed in an organ specific manner. In both thymic compartments, it appears that hematopoietic and non-hematopoietic cells contribute to T cell tolerance (52–58). Although T cell tolerance occurs at any stage in their development after rearrangement of a functional TCR (59, 60), the majority of T cell tolerance appears to occur in the thymic medulla (reviewed in (61, 62)). We discuss here our current understanding of how the array of medullary APC subsets contributes to thymic T cell tolerance.
mTEC antigen presentation
it remains technically challenging to delineate the specific contributions of each medullary APC to T cell tolerance development (1). For instance, reduction or deletion of MHC II on either compartment results in only a partial effect on Treg cell numbers or on negative selection (58, 63). Additionally, mTECs require crosstalk with thymocytes via MHC class II for successful organization of the medulla (64), precluding targeted deletion of MHC class II on mTECs. An important advance came from Klein and colleagues who developed a mouse with mTEC-targeted reduction of MHC class II expression using Aire promoter driven shRNA knockdown of the class II transactivator (C2TAkd) (58), which does so without altering medulla organization. They observed a modest increase in the polyclonal CD4SP compartment due to loss of negative selection as well as increased Treg cell selection to a model antigen expressed in mTECs. Thus, these data demonstrate that direct mTEC antigen presentation plays a role in T cell tolerance (Figure 2).
Figure 2.
mTECs, however, are known to be inefficient at using conventional antigen presenting pathways (65, 66). Moreover, conventional pathways are not focused on presenting proteins generated endogenously within the cell. Current data suggests that mTECs use macroautophagy for presentation of cell-autonomous antigens (67, 68). Transplantation of Atg5−/− autophagy-deficient thymi under the kidney capsule of select TCR transgenic mice resulted in increased CD4SP numbers, suggesting decrease negative selection. In addition, T cells selected on Atg5−/− thymi were capable of inducing multi-organ autoimmunity (67). Recent work suggested that mTECs specifically require autophagy to load peptides onto MHC class II from model antigens targeted to the autophagosome or the mitochondria, but not the cell membrane (68). These data on mTECs are consistent with reports from other contexts showing a role for autophagy in loading of intracellular antigen onto MHC class II (69–73).
However, another group reported that mTEC-targeted deletion of Atg7 via the K14-Cre resulted in no alteration of the thymocyte compartment or autoimmune disease despite ablation of autophagy in TECs (74). One possible explanation for the discrepancy between results from Atg5 versus Atg7 deficient mice is that autophagy associated antigen presentation may utilize different components than the autophagy pathway itself (71, 75–77). Interestingly, a recent report suggested that knockdown of Clec16a, an autophagy/mitophagy-associated protein (78, 79), is protective in the Non-obese diabetes (NOD) model of type 1 diabetes (80). They proposed that this was due to alterations in the generation and presentation of ligands by mTECs responsible for selecting diabetogenic T cells, rather than tolerance to islet associated antigens. Additional studies are required to determine whether an effect on mTEC antigen presentation explains the observation that an CLEC16A SNP results in increased susceptibility to human type 1 diabetes (81).
In summary, these data suggest that direct mTEC presentation of intracellular antigens to T cells is mediated at least in part by autophagy proteins. Future work is required to dissect the specific mechanism by which intracellular antigens are processed via the canonical autophagy versus alternative antigen presentation pathways in mTECs. Moreover, future analysis of TCR repertoires in mice with autophagy deficient mTECs would be useful to determine the extent that autophagy affects mTEC antigen presentation.
BM APCs
Whereas mTECs arise within the thymus, the other major medullary APC subsets arise from the bone marrow. Several lines of evidence suggest an important role for BM APCs in T cell tolerance. First, deletion of MHC class II on BM APCs results in an increase in the CD4SP thymocyte compartment (58, 63, 82), suggestive of a role in negative selection. The impact of MHC II deletion on BM APCs on Treg cell numbers have been reported to be mild (58, 63, 82–84) to moderate (85). Second, use of neo-self antigen systems such as RIP-mOVA suggest that BM APCs, and in particular DCs, can contribute to both negative and Treg cell selection of antigen-specific TCRs (52, 56, 86, 87).
Cooperation between BM APCs and mTECs
Antigen transfer from mTECs to BM APCs constitutes another pathway through which T cell tolerance occurs (9, 54, 55, 58, 88, 89). It is apparent that certain neo-self antigen transgenic models, such as nuclear or membrane-bound ovalbumin (OVA), require transfer from mTECs to thymic DCs for deletion of OVA-specific CD4+ and CD8+ thymocytes (55, 63). Antigen transfer was also reported by a separate group, although they also observed a concurrent role for cell-autonomous antigen presentation (52). In addition to model antigens, Anderson and colleagues examined this issue using MHC II tetramers with interphotoreceptor retinoid-binding protein (IRBP), a naturally occurring Aire-dependent peripheral tissue antigen (PTA) (54). Consistent with the notion that IRBP undergoes cooperative antigen presentation, they observed that deletion of MHC class II on BM APCs resulted in a significant increase in tetramer-positive cells, despite IRBP expression being restricted to mTECs.
Studies of medullary APC subsets typically examined individual TCR specificities or their effects on polyclonal T cell frequency. We therefore asked how BM APCs and mTECs shape the conventional and regulatory TCR repertoires using deep sequencing of the TCRα gene TRAV14 in a fixed TCRβ model (89). The fixed TCRβ model permits analysis of the TCR repertoire at the individual TCR level via analysis of the TCRα chain. Furthermore, we restricted the TCRα chain to a single functional locus preventing the potential confounder of secondary TCR rearrangement with dual TCR expression. We then genetically ablated MHC class II on BM APCs or reduced MHC II on mTECs via the C2TAkd strategy discussed above. We found the largest effect on CD4+ conventional T cells when MHC class II was absent in BM APCs, with approximately 20% versus 10% of the unique TCR repertoire dependent on BM APCs and mTECs, respectively. Note that the decrease of MHC II on mTECs using C2TAkd is only partial (58), and thus the role of mTECs is likely underestimated in this analysis. We observed a similar phenomenon in the Treg cell subset, with approximately 30% of the unique TCR repertoire dependent on BM APCs for negative selection of Treg cells, versus ~5% on mTECs. Importantly, we found that the Tconv and Treg cell TCR repertoires deleted by BM APCs and mTECs were distinct, demonstrating their non-redundant roles in T cell tolerance.
We were also able to determine if BM APCs and mTECs contributed to the selection of Treg cells (89). Similar to their effect on negative selection, both medullary APC subsets select different Treg cell TCR repertoires, contributing to around ~30–40% of the unique Treg TCR repertoire. Retroviral expression of several of these TCRs on thymocytes in vivo allowed us to confirm these TCR sequencing results as well as show that dendritic cells were the primary BM APC subset. In summary, the TCR repertoire analyses along with previous studies of TCR transgenic and polyclonal cells support the notion that cooperation between BM APCs and mTECs plays a substantial role in the deletion and Treg cell selection of developing thymocytes (Figure 2).
Role of Aire in T cell tolerance
A key component of tolerance is the presentation of self-antigens in the thymus. It has become clear that the Autoimmune Regulator (Aire) protein plays a critical role in this process by inducing the expression of tissue specific antigens in mTECs as well as mTEC function and development (reviewed in (62, 90–92)).
Control of tissue specific antigen production by Aire
It is clear that generation of PTA is one of the main functions of Aire (93). As an mTEC matures into the CD80+ MHC II+ phenotype, Aire expression becomes detectable which coincides with a dramatic increase in PTA expression. Despite the ability of the mTEC population to produce thousands of unique PTAs, previous reports estimate that a single mTEC expresses only ~1–3% of the total PTA ligandome (92). How a small subset of cells expressing a fraction of the possible PTA ligandome at any given time could facilitate tolerance remains unclear. Two recent studies utilizing single cell RNA-sequencing (scRNA-seq) address this complex process (94, 95). Analysis of ~200 individual mature mTECs confirmed previous estimates showing that each mTEC expressed only a small fraction of the total PTA ligandome. Combined, however, mTECs expressed the majority of known PTAs. Interestingly, both studies found that correlated sets of PTAs were generated in multiple distinct, small fractions of mTECs, providing evidence for a mosaic model of PTA expression (94, 95). Additionally, these “co-expressed genes” tended to cluster in the genome (95), providing a potential explanation for why a set of mTECs express a small, related fraction of PTAs at a particular time. Taken together, it appears that subsets of mature mTECs express clusters of PTAs and together these clusters of mTECs converge to express the breadth of the PTA ligandome.
Recently, Takayanag and colleagues have identified the transcriptional regulator Fezf2 as an Aire-independent mechanism for inducing PTA production in mTECs (96). Unlike Aire, deletion of Fezf2 is lethal in young (~3–4 week old) mice due to a failure of forebrain neuronal development and subsequent malnutrition (97). Moreover, Fezf2 is regulated by the lymphotoxin receptor β, whereas Aire is affected by the RANK/CD40 pathway. TEC-specific deletion of Fezf2 via Foxn1-cre results in lymphocytic infiltrates in the lung, liver, kidneys and small intestine but not the retina or pancreas of C57BL/6 mice, consistent with Fezf2 being required for expression of a different array of PTAs than Aire (96). The discovery of Fezf2 poses interesting new questions in its relationship to Aire and its relevance in human disease. Nonetheless, it appears that PTA expression in mTECs can be generated via multiple mechanisms.
The small numbers of mTECs that express any given PTA raises the question of how thymocytes are efficiently exposed to those antigens. One hypothesis is that the motility of thymocytes could allow them to scan hundreds to thousands of APCs over the period of time it resides in the medulla (1). Given the findings above, encountering ~200–500 mTECs would be sufficient for a single thymocyte to see the majority of the PTA ligandome. Another non-mutually exclusive possibility, as discussed in the preceding section, is that antigen transfer to BM APCs can increase the overall presentation of a given antigen.
Early studies supported the notion that Aire-induced PTA facilitates negative selection (54, 98, 99). However, its role in Treg cell selection has been controversial. Initial reports suggested that Aire is not required for Treg cell selection as Aire deletion does not alter polyclonal Treg cell numbers (98). Additionally, Ignatowicz and colleagues reported that the absence of Aire has no effect on the Treg cell TCR repertoire (100). On the other hand, Treg cell selection of TCR transgenic cells can be induced by the genetic expression of cognate antigen on Aire+ mTECs (63). Furthermore, a recent report by Savage and colleagues showed that two independent prostate specific TCR transgenic cells underwent Aire-dependent Treg cell selection (20). Together, these data suggest that Aire play a role in negative selection and can affect Treg cell selection at least in a limited capacity.
We also examined the effect of Aire on the Tconv and Treg TCR repertoire in our fixed TCRβ model (89). Aire was required for deletion of both Tconv and Treg cells, affecting about ~6–7% of the unique TCR repertoire, which is similar to that of MHC II reduction on mTECs using C2TAkd (~5%). By contrast, Aire was required for a substantial portion (~30%) of the unique Treg TCR repertoire. Interestingly, our Morisita-Horn similarity analysis showed substantial similarity between the Aire-sufficient and -deficient Treg cell repertoires, consistent with the previous TCR analysis (100). However, we noted that a handful of clones accounted for the similarity, masking the differences in lower frequency clones noted above (89). We confirmed six TCR clones showing Aire-dependence by TCR repertoire analysis using in vivo assays of Treg cell selection. Thus, our data support the notion that Aire is involved in Treg cell selection (20).
By cross-referencing our BM APC and C2TAkd TCR repertoire data with the Aire data, we were able to generate estimates of cooperative antigen presentation of Aire-dependent antigens (89). Approximately 3% of Tconv cells were co-dependent on BM APCs and Aire, suggesting that these TCRs were dependent on antigen transfer from mTECs to BM APCs for their deletion. A smaller percentage (1%) of unique TCRs was co-dependent on C2TAkd and Aire, consistent with cell-autonomous antigen presentation. Similar results were observed with analyses of the Treg cell data. Thus, our findings support and extend previous data suggesting that mTECs contribute to Treg cell selection via both cell-autonomous and cooperative antigen presentation of Aire-dependent antigens. Moreover, these data suggest that cooperative antigen presentation is not a rare process, but a primary pathway for tolerance induction.
The data raise additional questions regarding the nature of the antigens that are transferred versus presented endogenously by the mTEC, two non-mutually exclusive possibilities. RIP-mOVA is a cell surface protein that has been shown to be transferred to BM APCs, perhaps via transfer of membrane fragments–“cross-dressing” (101). Intracellular contents may be shunted into the autophagy pathway allowing for loading of endogenous peptide onto MHC class II within the phagolysosome. On the other hand, exosomes and apoptotic bodies may allow transfer of both cell surface and cytoplasmic proteins to BM APCs.
As discussed above, the rationale for antigen transfer from mTECs might be to expand the number of APCs presenting any given antigen considering the stochastic nature of Aire-dependent PTA expression. This would increase the likelihood that a single thymocyte can encounter all PTAs expressed in the thymus, and functionally increase the “antigenic niche size” for Treg and negative selection. Another intriguing possibility is that transfer to BM APCs insures that the processing and presentation of antigens are consistent between the thymus and periphery. For example, cathepsins are differentially expressed between TECs and BM APCs (102–105), potentially resulting in the loading of different peptide fragments onto the MHC II of these APC subsets. Although this hypothesis has not been tested, a dichotomy between thymic and peripheral antigen presentation has been hypothesized as a determining factor for the loss of tolerance to insulin in NOD mice (106). Thus, cooperative antigen presentation may limit discordant antigen peptide presentation by thymic versus peripheral APCs.
Alternative roles for Aire in mTEC function
The TCR repertoire data imply that the effect of Aire on the TCR repertoire is due to its ability to induce PTA. However, Aire affects many other aspects of mTEC biology. For instance, Aire can affect mTEC production of chemokines such as CCL17, CCL19, CCL22 and CCL25 involved in thymocytes trafficking from the cortex to the medulla (107, 108). Aire also induces mTECs to express XCL1 (108), a chemokine that recruits resident XCR1+-expressing CD8α+ DC and facilitates thymic Treg cell generation (108). Additionally, mTECs may produce the chemokines CCL2 (109, 110) and CCL25 (111), involved in the thymic entry of the migratory Sirpα+ DCs and plasmacytoid DCs (pDC), respectively. Thus, it remains possible that changes in the TCR repertoire due to Aire-deficiency may be related to recruitment of thymocytes and APCs to the thymic medulla.
Aire also appears to play an important role in mTEC development and maturation (112–115). Aire may be involved in apoptosis of mature mTEC cells (116). However, two recent reports have called into question the idea that Aire is pro-apoptotic (115, 117). In both, bacterial artificial chromosomes were used to introduce a tamoxifen-inducible Aire-Cre allowing for lineage tracing of developing mTECs. Both groups found that a post-Aire state of mTEC development occurred, during which Aire production is stopped, MHC class II and CD80/CD86 are down-regulated, and peripheral tissue antigens (PTA) transcript production is markedly diminished. These post-Aire mTECs reside for up to one week after reduction of Aire, and segregate towards the center of the medulla. Interrupting the process of mTEC maturation may affect the generation and transfer of antigens independent of PTA generation. Future studies are required to discriminate these multiple mechanisms by which Aire affect tolerance to self-antigens.
Importance of Aire-dependent tolerance early in ontogeny
The importance of Aire in medullary organization and PTA generation would suggest that the expression of Aire is important throughout life to enforce central tolerance. Remarkably, it has been shown that Aire expression is only required up to the end of the perinatal period to prevent autoimmunity in the NOD background (118). Recently, Mathis and colleagues explored the requirement for Aire in early ontogeny. Deletion of Treg cells via diphtheria treatment of Foxp3-DTR mice during 0 to 10 days after birth led to disease phenotypically similar to Aire-deficient mice though with substantially more general inflammation such as enlarged spleen and lymph nodes (119). Rescue of these Treg-depleted mice required Treg cells from 20 day old Aire-sufficient, but not -deficient, donors, consistent with a role for Aire in Treg cell selection.
To label Treg cells generated during the perinatal versus adult period of life, the authors injected tamoxifen into young or adult Foxp3eGFP-Cre-ERT2 Rosa 26-YFP mice. Interestingly, rescue of autoimmunity in Aire-deficient mice required Treg cells generated during the perinatal but not adult period of life. These perinatal generated Treg cells appear to be distinct transcriptionally from adult labelled Treg cells with heightened suppressive capacity in vitro. They also had distinct TCR repertoires from adult Treg TCRs. However, this did not appear to result from differences in PTAs induced by Aire in perinatal mice. Instead, the authors suggest that perinatal mTECs have a higher DM to DO ratio altering the antigen presentation machinery. Another possibility is that thymic cDCs are sparse during the perinatal period, potentially causing a difference in TCR repertoire independent of DM/DO expression. Why the lack of antigen transfer would enhance the tolerogenic potential of perinatal Treg cells, however, is unclear. Finally, it remains possible that peripheral expansion of perinatal Treg cells (120) may generate a population of “memory” Treg cells (121) with a different TCR repertoire and heightened Treg cell function compared with Treg cells generated as an adult. The presence of pre-existing Treg cells in the adult, but not perinatal, period may limit the activation of recent Treg cell thymic emigrants. Thus, multiple mechanisms could explain the differences in TCR repertoire and function between perinatal- versus adult-generated Treg cells.
BM-derived APC subsets
There are a variety of different BM-derived APC subsets in the thymic medulla, including B cells, macrophages, and DCs, consisting of conventional DCs and pDCs (122), each with potentially unique roles in thymic T cell tolerance.
Conventional DCs
The conventional DC subset is comprised of the CD8α+ and SIRPα+ DC subsets (123). It remains to be seen whether these thymic DC subsets can be further subdivided, such as that seen in the peripheral tissue SIRPα+ DC equivalent, the CD11b+ DCs (124). While these two cDC subsets are both efficient at presenting exogenous antigen, there are notable difference. For instance, thymic SIRPα+ DCs originate from outside of the thymus, presumably entering the thymus via the vascular plexus connected to the outer portion of the medulla (122, 123). By contrast, CD8α+ DCs arise primarily within the thymus. SIRPα+ DCs also show a different localization, and generally remain close to vascular regions within the medulla whereas CD8α+ DCs are distributed throughout the thymus parenchyma (1).
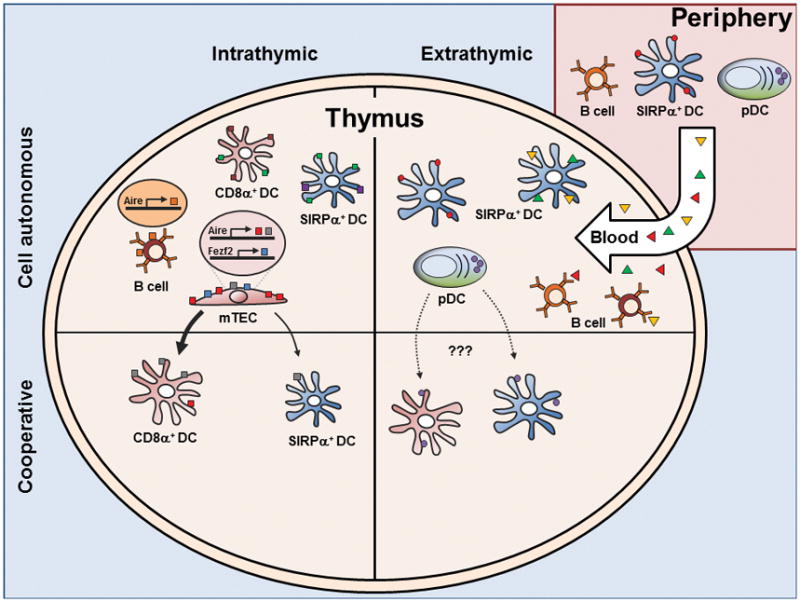
These differences suggest non-overlapping functions in antigen presentation (125). For instance, SIRPα+ DCs have been shown to efficiently present blood-borne antigen for negative and Treg cell selection, potentially related to their predilection for vascular regions (Figure 2) (56, 57, 65, 86).
The extra-thymic origin of SIRPα+ DCs may also be a vehicle for antigen transport into the thymus. One study using fluorescent beads too large to permeate into the thymus showed that SIRPα+ DCs and pDCs, but not CD8α+ DCs, have the capacity to acquire and transport antigen into the thymus (126). Similar findings were reported using skin-painting of Fluoroscein Isothiocyanate (FITC) (127). Finally, mOVA expressed specifically in cardiomyocytes led to deletion of OT-II thymocytes mediated by circulating DCs (127). Taken together, SIRPα+ DCs may present extra-thymic antigen both via DC migration as well as capture of blood-borne antigens (Figure 2).
Factors that determine if a SIRPα+ DC enters the thymus with extra-thymic antigen remain unclear. In addition, the relative importance of this extra-thymic versus intra-thymic antigen presentation is unknown. Another question is whether the extra-thymic antigen presentation pathway can induce tolerance to pathogens or commensal bacteria. Unfortunately, thymic SIRPα+ DCs are not affected by genetic manipulation such as CD11c-Cre Notch2fl/fl (128) or huLangerin-DTA (129) mice (Perry, J. S. A. and Hsieh, C.-S., unpublished data), in contrast with their effects on the peripheral DC population. New tools are therefore required to understand the importance of SIRPα+ DCs and the extent that they present extra- versus intra-thymic antigens.
Unlike SIRPα+ DCs, CD8α+ DCs are more likely to present antigen generated within the thymus because of their localization (Figure 2, reviewed in (130)). CD8α+ DCs constitutively express the chemokine receptor XCR1 (131) which directs migration in response to XCL1 from mTECs (108). This crosstalk between CD8α+ DCs and mTECs is supported by the observation that XCL1-deficient mice show a decrease in Treg cell numbers and transfer of thymocytes from XCL1 deficient mice into nude mice develop dacryoadenitis (108). Moreover, the enhanced ability of CD8α+ DCs to cross-present exogenous antigen on MHC I allows concomitant CD8+ T cell tolerance, as observed in both in vitro (132) and in vivo studies (55).
Given these clues, we hypothesized that CD8α+ DCs might be involved in selection of TCR clones that are co-dependent on Aire and BM APC antigen presentation. Indeed, four TCR clones that were co-dependent on BM APCs and Aire required CD8α+ DCs for Treg cell selection (89). By contrast, four TCRs that were BM APC-dependent but not Aire-independent were unaffected by the absence of CD8α+ DCs. While these data do not exclude a role for other BM APCs, it appears that CD8α+ DCs are important mediators of Aire-dependent, mTEC-mediated cooperative antigen presentation.
These data therefore demonstrate non-redundant roles for CD8α+ and SIRPα+ DCs in thymic tolerance. However, a number of questions remain. Deletion of thymic CD8α+ DCs utilized germline knockouts of the Basic Leucine Zipper Transcription Factor, ATF-like 3 (Batf3) (133). Although the currently available evidence suggests that loss of Treg cell selection is due to loss of the CD8α+ DC population in these mice, it remains possible that this represents a currently unknown function of Batf3 in DCs. Another question is whether CD8α+ DCs are the only DC subset involved in antigen transfer, or simply that it represents the dominant subset. Our data suggest that cytoplasmic material in mTECs, marked by GFP, can also be transferred to SIRPα+ DCs, albeit to a lesser degree (89). Finally, it would be of interest to determine the extent that antigen transfer drives CD8+ T cell tolerance, as that would be facilitated by the ability of CD8α+ DCs to cross-present exogenous antigens.
Plasmacytoid DCs
pDCs are also able to acquire and traffic peripheral antigen into the thymus, as assessed by fluorescently labeled particles injected into the periphery (126). pDCs are thought of as relatively poor APCs compared with cDCs (135, 136), yet there is evidence that pDCs participate in thymic tolerance. pDCs can present antigen to and promote the development of Treg cells in vitro (137), and injection of pDCs loaded with OVA can facilitate deletion of OT-II thymocytes which is abrogated in CCR9-deficient pDCs (126). The expression of CCR9 (126) might allow pDCs to migrate from the gut to the thymus with commensal antigens (1), but this hypothesis is as yet untested. Thus, while the proof of principle exists that pDCs can bring into the thymus antigens from the periphery to induce tolerance, the overall importance of extra-thymic antigen presentation by pDCs remains to be determined (Figure 2).
We performed a limited study of pDCs utilizing the CLEC4C-HBEGF (aka BDCA2-DTR) mouse in which pDCs can be selectively deleted upon diphtheria injection (138). We did not observe any effect of pDC depletion on Treg cell selection of several BM APC dependent TCRs (89). However, caveats of this study include the limited number of TCRs, and the possibility that pDCs could release antigen into the thymus during their deletion with diphtheria toxin. A combination of genetic deletion of pDCs with TCR repertoire analysis could be useful to test the hypothesis that pDCs may function to broaden thymic tolerance by presentation of extra-thymic antigens.
Thymic B Cells
Similar to pDCs, thymic B cells are relatively poor at acquiring soluble antigen. However, B cells are efficient at capturing antigen through their B cell receptor (BCR) (139). Thymic B cells are found at similar frequency to thymic DCs and can develop either intrathymically (140) or migrate from the periphery (141). Thymic B cells are unique from peripheral B cells in that they express high levels of MHC class II and various co-stimulatory molecules. Several reports suggest that thymic B cells are required for deletion and Treg cell selection (summarized in (141)) by providing co-stimulation via CD5-CD72 (142) or CD40-CD40L (49, 50, 141, 143), or by MHC-dependent interactions (50, 141, 144). Studies of a BCR transgenic specific for GPI showed that thymic B cells acquire this circulating self-antigen and delete cognate specific TCR transgenic cells (140) (Figure 2).
A recent report suggested that B cells may play a role in T cell tolerance independent of BCR specificity by presenting endogenous self-antigen (141). These cells enter from the periphery as naive B cells and are stimulated or “licensed” by T cells and CD40:CD40L to increase MHC class II, CD80, and Aire, which was expressed in about 50% of thymic B cells. In this model, BCR activation prevents this licensing process, arguing against a role for BCR specificity. Rather, the induction of Aire results in the expression and MHC presentation of self-antigens in B cells that are different from those in mTECs, thereby expanding the self-peptide:MHC repertoire presented in the thymus (Figure 2).
Future studies will be required to quantify the relative contribution of BCR specific versus Aire-dependent tolerance mediated by thymic B cells. Circulation of memory B cells to the thymus may result in efficient antigen presentation of soluble foreign antigens due to their BCR specificity. However, while BCR specificities to self might be expected to promote tolerance, BCRs specific to foreign antigens may result in immunodeficiency to those antigens. Finally, the relative importance of Aire-dependent antigens in B cells versus mTECs in T cell tolerance remains to be quantified. Thus, a number of questions remain regarding the role of B cells in T cell tolerance.
Conclusions and future directions
There have been a number of recent advances in our understanding of how self-reactive thymocytes are deleted or selected to become Treg cells. Recent data suggest that the medullary APCs subsets often function non-redundantly. pDCs and SIRPα+ DCs appear more adept at acquiring and presenting peripheral and blood-borne antigen, whereas mTECs and B cells present different arrays of Aire-dependent peripheral tissue antigens. Recent data also highlight the cooperativity between the APC subsets. Batf3-dependent CD8α+ DCs appear to preferentially acquire antigen from Aire+ mTECs to facilitate the development of thymic Treg cells. In fact, cooperative antigen presentation of mTEC antigens transferred to BM APCs may be as important as cell-autonomous mTEC antigen presentation.
However, a number of issues remain. First, identification of naturally occurring Treg cell TCR ligands would be useful to address which APCs generate and present these ligands and to determine the TCR affinity required for Treg and negative selection. Second, quantification of the contribution of each APC subset, as well as the importance of transport of extra-thymic antigens by migratory APC populations, to Treg and negative selection is needed. Third, understanding the factors that determine the utilization of mTEC-autonomous versus cooperative antigen transfer pathways for tolerance would be important for understanding why and how antigen transfer occurs. Finally, it is important to examine whether these mechanisms are regulated during ontogeny such that certain periods of life favor tolerance versus immunity. Thus, future studies will seek to define the interaction of the thymic antigenic repertoire with the APC subsets and the generation of thymic tolerance.
Acknowledgments
C.S.H. is supported by NIH NIAID AI079187 and the Burroughs Wellcome Fund; J.S.A.P. is the recipient of an R01 Research Supplement to Promote Diversity in Health-Related Research, NIH NIAID AI079187-06S1.
Footnotes
The authors declare no potential conflict of interest.
References
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014 doi: 10.1038/nri3667. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stritesky GL, Jameson SC, Hogquist KA. Selection of Self-Reactive T Cells in the Thymus. Annual Review of Immunology. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrisekoop N, Monteiro João P, Mandl Judith N, Germain Ronald N. Revisiting Thymic Positive Selection and the Mature T Cell Repertoire for Antigen. Immunity. 2014;41:181–190. doi: 10.1016/j.immuni.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nature Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 5.Klein L, Jovanovic K. Regulatory T cell lineage commitment in the thymus. Seminars in Immunology. 2011;23:401–409. doi: 10.1016/j.smim.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Rudensky AY. Regulatory T cells and Foxp3. Immunological Reviews. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Powrie F. Emerging Challenges in Regulatory T Cell Function and Biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 8.Klein L. Dead man walking: how thymocytes scan the medulla. Nature Immunol. 2009;10:809–811. doi: 10.1038/ni0809-809. [DOI] [PubMed] [Google Scholar]
- 9.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. The Journal of Experimental Medicine. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gascoigne NR, Palmer E. Signaling in thymic selection. Curr Opin Immunol. 2011;23:207–212. doi: 10.1016/j.coi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 12.Leung MWL, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. The Journal of Experimental Medicine. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivares-Villagómez D, Wang Y, Lafaille JJ. Regulatory CD4+ T Cells Expressing Endogenous T Cell Receptor Chains Protect Myelin Basic Protein–specific Transgenic Mice from Spontaneous Autoimmune Encephalomyelitis. The Journal of Experimental Medicine. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh M, et al. Thymus and Autoimmunity: Production of CD25+CD4+ Naturally Anergic and Suppressive T Cells as a Key Function of the Thymus in Maintaining Immunologic Self-Tolerance. The Journal of Immunology. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 15.Moon JJ, et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3− subsets of self-peptide/MHC class II-specific CD4+ T cells. Proceedings of the National Academy of Sciences. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh C-S, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 17.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR Repertoires to Self-Peptides in Regulatory and Nonregulatory CD4+ T Cells. The Journal of Immunology. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 18.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of Experimental Medicine. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bautista JL, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malchow S, et al. Aire-Dependent Thymic Development of Tumor-Associated Regulatory T Cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H-M, Bautista Jhoanne L, Scott-Browne J, Mohan James F, Hsieh C-S. A Broad Range of Self-Reactivity Drives Thymic Regulatory T Cell Selection to Limit Responses to Self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beringer DX, et al. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat Immunol. 2015;16:1153–1161. doi: 10.1038/ni.3271. [DOI] [PubMed] [Google Scholar]
- 23.Wojciech L, et al. The same self-peptide selects conventional and regulatory CD4+ T cells with identical antigen receptors. Nat Commun. 2014;5 doi: 10.1038/ncomms6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hood JD, Zarnitsyna VI, Zhu C, Evavold BD. Regulatory and T Effector Cells Have Overlapping Low to High Ranges in TCR Affinities for Self during Demyelinating Disease. The Journal of Immunology. 2015;195:4162–4170. doi: 10.4049/jimmunol.1501464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen P, et al. Discrete TCR Repertoires and CDR3 Features Distinguish Effector and Foxp3+ Regulatory T Lymphocytes in Myelin Oligodendrocyte Glycoprotein-Induced Experimental Allergic Encephalomyelitis. The Journal of Immunology. 2010;185:3895–3904. doi: 10.4049/jimmunol.1001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, et al. T Cell Receptor CDR3 Sequence but Not Recognition Characteristics Distinguish Autoreactive Effector and Foxp3+ Regulatory T Cells. Immunity. 2009;31:909–920. doi: 10.1016/j.immuni.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lio C-WJ, Hsieh C-S. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinterberger M, Wirnsberger G, Klein L. B7/CD28 in central tolerance: costimulation promotes maturation of regulatory T cell precursors and prevents their clonal deletion. Frontiers in immunology. 2011;2 doi: 10.3389/fimmu.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burchill MA, et al. Linked T Cell Receptor and Cytokine Signaling Govern the Development of the Regulatory T Cell Repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, et al. Conversion of Peripheral CD4+CD25− Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. The Journal of Experimental Medicine. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MO, Sanjabi S, Flavell Richard A. Transforming Growth Factor-β Controls Development, Homeostasis, and Tolerance of T Cells by Regulatory T Cell-Dependent and -Independent Mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-[beta] signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 33.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 34.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but Not Thymic Stromal Lymphopoeitin, Redundantly Govern CD4+Foxp3+ Regulatory T Cell Development. The Journal of Immunology. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 Receptor β-Dependent STAT5 Activation Is Required for the Development of Foxp3+ Regulatory T Cells. The Journal of Immunology. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 36.Tai X, et al. Foxp3 Transcription Factor Is Proapoptotic and Lethal to Developing Regulatory T Cells unless Counterbalanced by Cytokine Survival Signals. Immunity. 2013;38:1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 38.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015;16:635–641. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 Secretion by CD4+ T Cells In Vivo Is Rapid, Transient, and Influenced by TCR-Specific Competition. The Journal of Immunology. 2004;172:6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- 40.Thiault N, et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol. 2015;16:628–634. doi: 10.1038/ni.3150. [DOI] [PubMed] [Google Scholar]
- 41.Cebula A, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang E, Zou T, Leichner TM, Zhang SL, Kambayashi T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. European Journal of Immunology. 2014;44:2712–2720. doi: 10.1002/eji.201444529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Q, et al. Cutting Edge: CD28 Controls Peripheral Homeostasis of CD4+CD25+ Regulatory T Cells. The Journal of Immunology. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 44.Salomon B, et al. B7/CD28 Costimulation Is Essential for the Homeostasis of the CD4+CD25+ Immunoregulatory T Cells that Control Autoimmune Diabetes. Immunity. 12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 45.Lio C-WJ, Dodson LF, Deppong CM, Hsieh C-S, Green JM. CD28 Facilitates the Generation of Foxp3− Cytokine Responsive Regulatory T Cell Precursors. The Journal of Immunology. 2010;184:6007–6013. doi: 10.4049/jimmunol.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahmud SA, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15:473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, et al. Id1 Expression Promotes T Regulatory Cell Differentiation by Facilitating TCR Costimulation. The Journal of Immunology. 2014;193:663–672. doi: 10.4049/jimmunol.1302554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams JA, et al. Thymic Medullary Epithelium and Thymocyte Self-Tolerance Require Cooperation between CD28–CD80/86 and CD40–CD40L Costimulatory Pathways. The Journal of Immunology. 2014;192:630–640. doi: 10.4049/jimmunol.1302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuss SM, Green EA. Abrogation of CD40–CD154 Signaling Impedes the Homeostasis of Thymic Resident Regulatory T Cells by Altering the Levels of IL-2, but Does Not Affect Regulatory T Cell Development. The Journal of Immunology. 2012;189:1717–1725. doi: 10.4049/jimmunol.1200588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu F-T, et al. Thymic B cells promote thymus-derived regulatory T cell development and proliferation. Journal of Autoimmunity. 2015;61:62–72. doi: 10.1016/j.jaut.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Coquet JM, et al. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27–CD70 pathway. The Journal of Experimental Medicine. 2013;210:715–728. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hubert F-X, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 53.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taniguchi RT, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proceedings of the National Academy of Sciences. 2012;109:7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallegos AM, Bevan MJ. Central Tolerance to Tissue-specific Antigens Mediated by Direct and Indirect Antigen Presentation. The Journal of Experimental Medicine. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atibalentja DF, Murphy KM, Unanue ER. Functional Redundancy between Thymic CD8α+ and Sirpα+ Conventional Dendritic Cells in Presentation of Blood-Derived Lysozyme by MHC Class II Proteins. The Journal of Immunology. 2011;186:1421–1431. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Proietto AI, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proceedings of the National Academy of Sciences. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 59.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. The Journal of Experimental Medicine. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proceedings of the National Academy of Sciences. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 62.Anderson MS, Su MA. Aire and T cell development. Current Opinion in Immunology. 2011;23:198–206. doi: 10.1016/j.coi.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 64.Irla M, et al. Autoantigen-Specific Interactions with CD4+ Thymocytes Control Mature Medullary Thymic Epithelial Cell Cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183:7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein L, Roettinger B, Kyewski B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. European Journal of Immunology. 2001;31:2476–2486. doi: 10.1002/1521-4141(200108)31:8<2476::aid-immu2476>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 67.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 68.Aichinger M, Wu C, Nedjic J, Klein L. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J Exp Med. 2013;210:287–300. doi: 10.1084/jem.20122149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paludan C, et al. Endogenous MHC Class II Processing of a Viral Nuclear Antigen After Autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 70.English L, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee HK, et al. In Vivo Requirement for Atg5 in Antigen Presentation by Dendritic Cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dengjel J, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proceedings of the National Academy of Sciences. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid D, Pypaert M, Münz C. Antigen-Loading Compartments for Major Histocompatibility Complex Class II Molecules Continuously Receive Input from Autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sukseree S, et al. Autophagy in the Thymic Epithelium Is Dispensable for the Development of Self-Tolerance in a Novel Mouse Model. PLoS ONE. 2012;7:e38933. doi: 10.1371/journal.pone.0038933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Z, et al. Autophagosome-Independent Essential Function for the Autophagy Protein Atg5 in Cellular Immunity to Intracellular Pathogens. Cell Host & Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Ye Y, Zhou X, Huang C, Wu M. Atg7 Enhances Host Defense against Infection via Downregulation of Superoxide but Upregulation of Nitric Oxide. The Journal of Immunology. 2015;194:1112–1121. doi: 10.4049/jimmunol.1401958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu E, Van Grol J, Subauste CS. Atg5 but not Atg7 in dendritic cells enhances IL-2 and IFN-γ production by Toxoplasma gondii-reactive CD4+ T cells. Microbes and Infection. 2015;17:275–284. doi: 10.1016/j.micinf.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soleimanpour Scott A, et al. The Diabetes Susceptibility Gene Clec16a Regulates Mitophagy. Cell. 2014;157:1577–1590. doi: 10.1016/j.cell.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soleimanpour SA, et al. Diabetes susceptibility genes Pdx1 and Clec16a function in a pathway regulating mitophagy in β-cells. Diabetes. 2015 doi: 10.2337/db15-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuster C, et al. The Autoimmunity-Associated Gene CLEC16A Modulates Thymic Epithelial Cell Autophagy and Alters T Cell Selection. Immunity. 2015;42:942–952. doi: 10.1016/j.immuni.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011;12:781–792. doi: 10.1038/nrg3069. [DOI] [PubMed] [Google Scholar]
- 82.Ohnmacht C, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. The Journal of Experimental Medicine. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liston A, et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proceedings of the National Academy of Sciences. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Meerwijk JPM, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative Impact of Thymic Clonal Deletion on the T Cell Repertoire. The Journal of Experimental Medicine. 1997;185:377–384. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Román E, Shino H, Qin FX-F, Liu YJ. Cutting Edge: Hematopoietic-Derived APCs Select Regulatory T Cells in Thymus. The Journal of Immunology. 2010;185:3819–3823. doi: 10.4049/jimmunol.0900665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp[alpha]+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 87.Oh J, et al. MARCH1-mediated MHCII ubiquitination promotes dendritic cell selection of natural regulatory T cells. The Journal of Experimental Medicine. 2013;210:1069–1077. doi: 10.1084/jem.20122695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hubert FX. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 89.Perry Justin SA, et al. Distinct Contributions of Aire and Antigen-Presenting-Cell Subsets to the Generation of Self-Tolerance in the Thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mathis D, Benoist C. Aire. Annual Review of Immunology. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 91.Akirav EM, Ruddle NH, Herold KC. The role of AIRE in human autoimmune disease. Nat Rev Endocrinol. 2011;7:25–33. doi: 10.1038/nrendo.2010.200. [DOI] [PubMed] [Google Scholar]
- 92.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 93.Taniguchi RT, Anderson MS. The role of Aire in clonal selection. Immunol Cell Biol. 2011;89:40–44. doi: 10.1038/icb.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brennecke P, et al. Single-cell transcriptome analysis reveals coordinated ectopic gene-expression patterns in medullary thymic epithelial cells. Nat Immunol. 2015;16:933–941. doi: 10.1038/ni.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meredith M, Zemmour D, Mathis D, Benoist C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat Immunol. 2015;16:942–949. doi: 10.1038/ni.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takaba H, et al. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell. 2015;163:975–987. doi: 10.1016/j.cell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 97.Eckler MJ, Chen B. Fez family transcription factors: Controlling neurogenesis and cell fate in the developing mammalian nervous system. BioEssays. 2014;36:788–797. doi: 10.1002/bies.201400039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The Cellular Mechanism of Aire Control of T Cell Tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 100.Daniely D, Kern J, Cebula A, Ignatowicz L. Diversity of TCRs on Natural Foxp3+ T Cells in Mice Lacking Aire Expression. The Journal of Immunology. 2010;184:6865–6873. doi: 10.4049/jimmunol.0903609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakagawa T, et al. Cathepsin L: Critical Role in Ii Degradation and CD4 T Cell Selection in the Thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 103.Beers C, Burich A, Kleijmeer MJ, Griffith JM, Wong P, Rudensky AY. Cathepsin S Controls MHC Class II-Mediated Antigen Presentation by Epithelial Cells In Vivo. The Journal of Immunology. 2005;174:1205–1212. doi: 10.4049/jimmunol.174.3.1205. [DOI] [PubMed] [Google Scholar]
- 104.Bania J, et al. Human cathepsin S, but not cathepsin L, degrades efficiently MHC class II-associated invariant chain in nonprofessional APCs. Proceedings of the National Academy of Sciences. 2003;100:6664–6669. doi: 10.1073/pnas.1131604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stoeckle C, et al. Cathepsin S dominates autoantigen processing in human thymic dendritic cells. Journal of Autoimmunity. 2012;38:332–343. doi: 10.1016/j.jaut.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Current Opinion in Immunology. 2014;26:32–40. doi: 10.1016/j.coi.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 108.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. The Journal of Experimental Medicine. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cédile O, et al. Thymic CCL2 influences induction of T-cell tolerance. Journal of Autoimmunity. 2014;55:73–85. doi: 10.1016/j.jaut.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 110.Baba T, Nakamoto Y, Mukaida N. Crucial Contribution of Thymic Sirpα+ Conventional Dendritic Cells to Central Tolerance against Blood-Borne Antigens in a CCR2-Dependent Manner. The Journal of Immunology. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 111.Hadeiba H, et al. Plasmacytoid Dendritic Cells Transport Peripheral Antigens to the Thymus to Promote Central Tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG. Aire-Dependent Alterations in Medullary Thymic Epithelium Indicate a Role for Aire in Thymic Epithelial Differentiation. The Journal of Immunology. 2007;178:3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 113.Nishikawa Y, et al. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. The Journal of Experimental Medicine. 2010;207:963–971. doi: 10.1084/jem.20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yano M, et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. The Journal of Experimental Medicine. 2008;205:2827–2838. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishikawa Y, et al. Temporal Lineage Tracing of Aire-Expressing Cells Reveals a Requirement for Aire in Their Maturation Program. The Journal of Immunology. 2014;192:2585–2592. doi: 10.4049/jimmunol.1302786. [DOI] [PubMed] [Google Scholar]
- 116.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. The Journal of Experimental Medicine. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Metzger Todd C, et al. Lineage Tracing and Cell Ablation Identify a Post-Aire-Expressing Thymic Epithelial Cell Population. Cell Reports. 2013;5:166–179. doi: 10.1016/j.celrep.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. The Journal of Experimental Medicine. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh C-S. Antigen-specific peripheral shaping of the natural regulatory T cell population. The Journal of Experimental Medicine. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oh J, Shin J-S. The Role of Dendritic Cells in Central Tolerance. Immune Netw. 2015;15:111–120. doi: 10.4110/in.2015.15.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wendland M, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proceedings of the National Academy of Sciences. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 128.Satpathy AT, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–948. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyártó BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. The Journal of Experimental Medicine. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 131.Bachem A, et al. Expression of XCR1 Characterizes the Batf3-Dependent Lineage of Dendritic Cells Capable of Antigen Cross-Presentation. Frontiers in Immunology. 2012;3:214. doi: 10.3389/fimmu.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Proietto AI, Lahoud MH, Wu L. Distinct functional capacities of mouse thymic and splenic dendritic cell populations. Immunol Cell Biol. 2008;86:700–708. doi: 10.1038/icb.2008.63. [DOI] [PubMed] [Google Scholar]
- 133.Hildner K, et al. Batf3 Deficiency Reveals a Critical Role for CD8α+ Dendritic Cells in Cytotoxic T Cell Immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hadeiba H. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Villadangos JA, Young L. Antigen-Presentation Properties of Plasmacytoid Dendritic Cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 136.Steinman RM. Decisions About Dendritic Cells: Past, Present, and Future. Annual Review of Immunology. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 137.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proceedings of the National Academy of Sciences. 2009;106:10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid Dendritic Cell Ablation Impacts Early Interferon Responses and Antiviral NK and CD8+ T Cell Accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 140.Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proceedings of the National Academy of Sciences. 2013;110:17011–17016. doi: 10.1073/pnas.1313001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamano T, et al. Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction. Immunity. 2015;42:1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 142.Xing C, et al. Critical role for thymic CD19+CD5+CD1dhiIL-10+ regulatory B cells in immune homeostasis. Journal of Leukocyte Biology. 2015;97:547–556. doi: 10.1189/jlb.3A0414-213RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fujihara C, Williams JA, Watanabe M, Jeon H, Sharrow SO, Hodes RJ. T Cell–B Cell Thymic CrossTalk: Maintenance and Function of Thymic B Cells Requires Cognate CD40–CD40 Ligand Interaction. The Journal of Immunology. 2014;193:5534–5544. doi: 10.4049/jimmunol.1401655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walters SN, Webster KE, Daley S, Grey ST. A Role for Intrathymic B Cells in the Generation of Natural Regulatory T Cells. The Journal of Immunology. 2014;193:170–176. doi: 10.4049/jimmunol.1302519. [DOI] [PubMed] [Google Scholar]


