Iodine Test Definition
The iodine test is a starch identification test based on a chemical reaction. Iodine and starch generate a unique blue-black coloured complex in this test.
What does Iodine Test for?
The iodine test is a chemical reaction-based identification test for starch. This test helps to identify the presence of starch in a sample.
It also helps to distinguish between mono– or disaccharides from polysaccharides (glycogen, dextrin, and amylase). In this test, iodine and starch form a distinct blue-black colored complex.

What Color is a Positive Iodine Test?
A positive result for the iodine test (starch is present) was a colour change ranging from violet to black; a negative result (no starch) was the yellow colour of the iodine solution.
What was the Positive Controls of the Iodine Test procedure?
The positive control is starch solution.
What was the Negative Controls of the Iodine Test procedure?
The negative control is distilled water.
What is Iodine Test?
This test can be used to determine whether or not a sample contains starch. It also aids in the differentiation of mono- and disaccharides from polysaccharides (glycogen, dextrin, and amylase).
A test to see if there is any starch present. When a few drops of potassium iodide solution are poured on the sample, it turns blue-black. Polyiodide chains are formed as a result of the interaction between starch and iodine.
Iodine molecules assemble in helices formed by amylose in starch, resulting in a dark blue or black appearance. The blue-black hue is not formed when starch is broken down or hydrolyzed into smaller carbohydrate units. As a result, even if no colour change occurs, this test can suggest that hydrolysis is complete.
Objective of Iodine Test
The Iodine test was used to determine the presence of starch in the sample presented.
Iodine Test Principle
Let’s take a look at starch and its chemistry before we get into the theory behind the iodine test. A polysaccharide is starch. Plants store glucose in the form of starch. Potatoes and cereals contain a lot of starch (oats, barley, rice, wheat).
Amylose (10-20%) and Amylopectin (10-20%) are the two monomeric components found in natural starch (80-90 percent ). Both monomers are made up of D-glucose subunits chemically. The D-glucose unit in Amylose and Amylopectin, on the other hand, is positioned differently.
Amylose vs Amylopectin
| Amylose | Amylopectin |
| D-glucose subunits form a straight chain polymer. | D-glucose subunits form a branched-chain polymer. |
| Glucose units in Amylose are connected by -1,4 glycosidic bonds. | Glucose units in Amylopectin are linked together by -1,6 glycosidic connections. |
| Amylose makes up 20% of starch. | Amylopectin makes up 80% of starch. |
| Amylose is a water-insoluble starch component. | The water-soluble part of starch is called amylopectin. |
| When hot water is added to the mixture, it does not gel. | Gelling occurs when hot water is added. |
| With iodine solution, it turns blue. | Iodine solution does not produce a blue colour. |
Iodine Test Mechanism
The iodine test is based on the interaction of Amylose with starch to generate a blue-black coloured complex with the iodine. Amylose’s helical structure creates a charge transfer (CT) complex with iodine, which is found inside Amylose’s spiral or helical structure.
Iodine in water, i.e., an aqueous solution of molecular iodine (I) and potassium iodide (KI), also known as Lugol’s iodine, is used for this test. It’s worth noting that this is also referred to as the IKI solution.
I + KI = IKI Solution
Iodine molecule, also known as molecular iodine or I2, is insoluble in water. As a result, potassium iodide is utilised in the preparation of the laboratory reagent. Iodide ions are formed when potassium iodide dissociates.
In solution, iodide ions combine to generate triiodide ions (I3–), which then associate to make polyiodide ions (In–). The formation of In– ions is largely due to I3– chemistry.
Negatively charged polyiodide ions include triiodide (I3–), pentaiodide (I5–), and heptaiodide (I7–). Charge donors, these polyiodide ions form a combination with Amylose. Lugol’s iodine solution on the bench is brown in colour.
However, electrons absorb light energy and are stimulated to a higher energy level in the charge transfer complex of polyiodide ions and Amylose. The human eye perceives the complementary hue to the light energy absorbed by the charge transfer complex as a blue-black colour.
As a result, the iodine colour on the benchtop is brown. Colorless polyiodides (I3–, I5–, and I7–) and a blue-black amylose-iodide complex Because the Iodine starch reaction is unique, it is used to detect starch in a sample.
As a result, an IKI indicator can be used to certify the presence of starch. The iodine starch test’s premise is also the foundation for all iodometric titrations that use the starch indicator.
The intensity of the blue colour reduces as the temperature rises when water-miscible solvents such as isopropyl alcohol, ethanol, and others are present. Because the amylose-iodine combination dissociates when the temperature rises, this is the case.
The helical structure of the amylose-iodine complex is reconstructed when the temperature decreases, resulting in the regeneration of the blue-black colour complex
Iodine Test Reagents
You’ll need the following items to perform the starch identification test:
Iodine solution or Lugol’s reagent, test sample, Tubes for testing, Stand for test tubes, Vortex mixer in a water bath
What is Iodine Solution?
It’s a mixture of molecular iodine (5%) and potassium iodide in an aqueous solution (10 percent). Iodine solution is brown in colour and should be kept in a dark place.
Schematic of Iodine Test Procedure
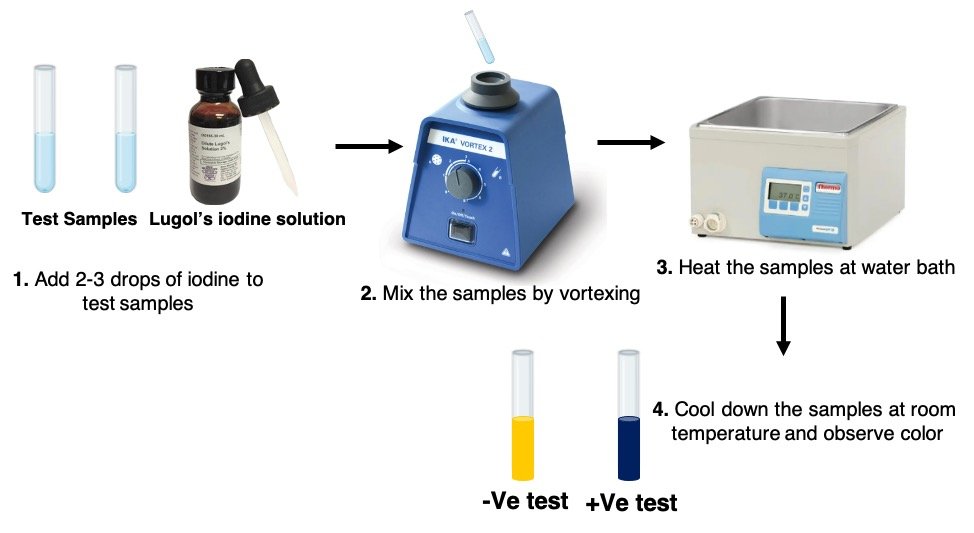
Iodine Test Procedure
The steps of the Iodine Test are as follows:
1. Label two test tubes with the words “test sample” and “control sample.”
2. In a clean and dried test tube designated as a test sample, take a small sample (solid sample: 500 mg-1000 mg; liquid sample: 1 ml).
3. In the clean and dried test tube designated as the control sample, pour 1 mL of purified water.
4. To both test tubes, add 2-3 drops of Lugol’s iodine solution and thoroughly mix with a vortex mixer.
5. Keep an eye on the colour changes in both test tubes.
6. After that, the test tubes should be heated in a water bath until the colour has faded.
7. Allow the test tubes to cool completely before examining the colour in both of them.
Iodine Test Results
• The presence of starch in the sample is shown by the emergence of a blue-black tint, indicating a positive iodine test.
• The lack of starch in the given sample, as indicated by the brown hue or no change in colour, indicates a negative iodine test.

Iodine Test Uses
• The presence of starch in a sample can be determined using an iodine test.
• Starch may be distinguished from monosaccharides, disaccharides, and other polysaccharides using the iodine test.
• To distinguish between starch, glycogen, and carbs, the iodine test is employed.
• Blood iodine testing is performed to determine whether a patient has hyperthyroidism or hypothyroidism.
• The starch hydrolysis test follows the same principles as the iodine test.
• The iodine test is the foundation for iodometric titrations that use a starch indicator.
Iodine Test Limitation
• One of the most significant drawbacks of the iodine test is that it is qualitative. That is, the presence or lack of starch in the sample can be detected. The iodine test, on the other hand, cannot be used to quantify the amount of starch present in the sample.
• Another drawback is that starch hydrolysis occurs under acidic circumstances. As a result, the iodine test will not work with acidic samples.
• The iodine test cannot be done on a sample that is extremely dark in colour because the colour changes will be undetectable.
Iodine Test Important Points
• Because Lugol’s iodine solution is light-sensitive, it should be kept in a dark amber-colored bottle in a dark location.
• The iodine test is a starch-specific characteristic test. Lugol’s iodine solution, for example, will not modify the colour of the cellulose.
• The test is susceptible to temperature changes.
Iodine Test Citations
Comparison of the Accuracy of Papanicolaou Test Cytology, Visual Inspection With Acetic Acid, and Visual Inspection With Lugol Iodine in Screening for Cervical Neoplasia in Southeast Nigeria. J Glob Oncol . 2018 Sep;4:1-9.
Share












