Chapter 8 Covalent Bonding NOTE The numbered sections

Chapter 8 Covalent Bonding NOTE: The numbered sections on this outline do NOT correspond to Chapter Sections in the book. 8. 1 - Covalent Bonding & Molecules Covalent Bond vs. Ionic Bond Molecule vs. Formula Unit Unshared Pair (Nonbonding Pair) Diatomic Molecules Double & Triple Bonds 8. 2 - Molecular Shape & Geometry VSEPR Theory Molecular Shapes 8. 3 - Bond Polarity & Intermolecular Attractions Electronegativity – Define & Periodic Trend (Review Nonpolar & Polar Covalent δ and +→ Notation van der Waals Forces Dipole Interactions, Hydrogen Bonding Dispersion Forces

8. 1 - Covalent Bonding & Molecules Ionic bonds are formed by electron transfer. (b/w metal & nonmetal) Covalent bonds are formed by electron sharing. (b/w 2 nonmetals)

Covalent bonds form molecules. single covalent bond H 2 double covalent bond O 2 Each atom shares e- with another to have an octet.

How are ionic bonds different from covalent? IONIC COVALENT transfer of e- (forms ions!) sharing of e- (COvalent) formula units molecules formed b/w metal & nonmetal formed b/w 2 nonmetals High melting points low melting points (most are gases or liquids at room temp)

IONIC Na. Cl COVALENT Array of sodium ions and chloride ions Collection of water molecules Formula unit of sodium chloride Molecule of water Chemical formula H 2 O
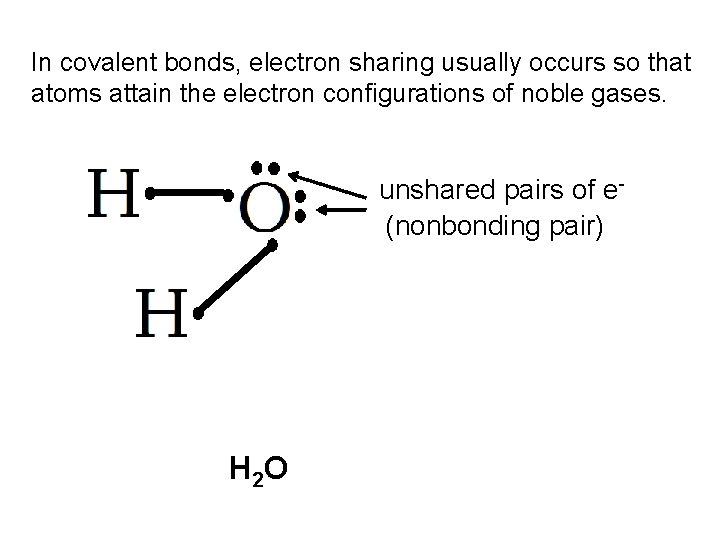
In covalent bonds, electron sharing usually occurs so that atoms attain the electron configurations of noble gases. unshared pairs of e(nonbonding pair) H 2 O

Diatomic Molecules - 2 identical atoms covalently bonded together. 7 diatomic molecules H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2

ASSIGN: Chapter 8 Worksheet #1

8. 2 - Molecular Shape & Geometry Valence Shell Electron Pair Repulsion Theory (VSEPR) electron pairs repel each other and stay far apart from each other. VSEPR explains the 3 D shapes of molecules.

Common Molecular Shapes (p. 243) Linear Trigonal planar Trigonal Pyramidal Tetrahedral Bent
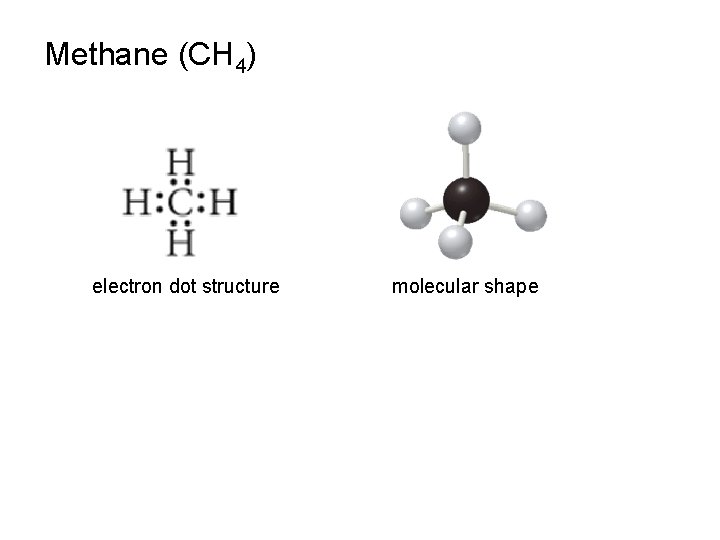
Methane (CH 4) electron dot structure molecular shape

Molecular Geometry – 3 d arrangement of bonded atoms INCLUDING nonbonding electron pairs Molecular Shape – 3 d arrangement of bonded atoms ONLY The molecular shape may not be the same as the geometry

Unshared pairs of electrons require space as well. Ammonia (NH 3) Unshared electron pair molecular geometry electron dot structure molecular shape

Water (H 2 O) Unshared pairs molecular geometry electron dot structure The water molecule has a bent shape because of the unshared pairs molecular shape

8. 1 - Covalent Bonding & Molecules Lewis Symbols for Elements 3 properties of Ionic & Covalent Bonds 7 Diatomic Molecules Electron Dot Structure for Molecules Non-bonding (Unshared) Electron Pairs) 8. 2 - Molecular Shape & Geometry Explain VSEPR Theory Molecular Shape vs. Molecular Geometry

8. 3 - Bond Polarity & Intermolecular Attractions Electronegativity – Define & Periodic Trend (Review Nonpolar & Polar Covalent Bonds and +→ Notation Polar & Nonpolar Molecules van der Waals Forces Dipole Interactions, Hydrogen Bonding Dispersion Forces Review from Chapter 6 Electronegativity – an atom’s attraction for electrons.

Electronegativity Review… B<H<C Noble gases do not have e-neg values

Covalent bonding involves a sharing of valence electrons. But, the sharing may not be equal.
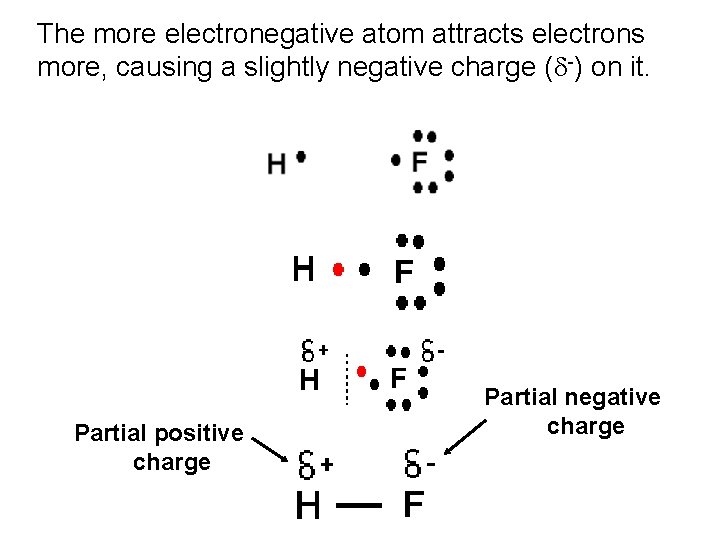
The more electronegative atom attracts electrons more, causing a slightly negative charge ( -) on it. Partial positive charge Partial negative charge

Polar Covalent Bond (polar bond) – covalent bond in which the electrons are shared unequally.

A nonpolar covalent bond occurs between 2 identical atoms. Since each atom in a nonpolar bond has the same electronegativity, the electrons are shared equally. 7 diatomic molecules (H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2)

The polar nature of a bond can also be shown by an arrow pointing to the more electronegative atom. H—Cl In our class, the bond between 2 atoms can only be 1) Ionic – b/w metal & nonmetal 2) Polar Covalent – b/w 2 different nonmetals 3) Nonpolar Covalent – b/w 2 identical nonmetals 4) Metallic – b/w 2 metals

Identify the bonds between these elements as ionic, polar covalent or nonpolar covalent. a) H – Br b) K – Cl c) C – O d) Li – O e) Cl – F f) Br – Br g) H – O h) H – Mg Place a - symbol above the more electronegative atom in the bond.

The polar bonds cause a molecule to be polar or nonpolar A polar molecule is also called a dipole

Carbon dioxide is a nonpolar molecule although it contains polar bonds. The polar bonds cancel out in this case.

Water is a polar molecule since its partial charges do not cancel out.

Intermolecular Attractions – attractive forces between separate molecules; also called van der Waals Forces These attractive forces are much weaker than ionic or covalent bonds, but without these attractions, solid and liquid matter would not exist.

1. Dipole Interactions – occurs when polar molecules are attracted to one another. 3 acetone molecules attracting each other.

Hydrogen Bond – strong intermolecular attractive force between H in one molecule and an electronegative atom in another nearby molecule. Hydrogen Bonds in H 2 O

hydrogen bond The slightly charged atoms on separate water molecules create an attractive force between them.

Cohesion – attraction to same substance. Adhesion – attraction to different substance



ASSIGN: Read Dispersion Forces (p. 251) 1. What is a dispersion force? 2. What causes dispersion forces between molecules? 3. Which molecules do dispersion forces occur in? 4. Which types of molecules are dispersion forces most important?

2. Dispersion Forces – temporary attractive forces between molecules due to random electron motion (London forces) F 2 melts at 53 K, Boils at 85 K

Without dispersion forces, nonpolar molecules could never be liquids or solids. Molecule Melt Pt Boil Pt F 2 53 K 85 K Cl 2 171. 6 K 239. 1 K Br 2 265. 8 K 332. 0 K I 2 386. 8 K 457. 4 K Cohesion – attraction to same substance. Adhesion – attraction to different substance

Chapter 8 ASSIGNMENT #29 -38 & 64 -68 page 256, 257
- Slides: 37



























































