Chemical Bonding Chapter 13 Electrons and Chemical Bonding

Chemical Bonding Chapter 13

Electrons and Chemical Bonding Have you ever stopped to consider that by using the 26 letters of the alphabet, you make all of the words you use every day? Although the number of letters is limited, you combine them in different ways to make a huge number of words. This is the same with elements. We combine them to make different substances.

Combining Atoms Through Chemical Bonding - is the joining of atoms to form new substances The ______ of these new substances are ______ than the _____ of the original substance. When chemical bonds form, _____ are ________, or _____.

Electron Number and Organization To understand how atoms form chemical bonds, you need to understand how ________ are arranged in an atom. The number of electrons in an atom = __________. The number of protons can be determined by the _______. The electrons are organized in _________. 1 st Energy Level: 2 nd Energy Level: 3 rd Energy Level: Diagram Fluorine:

Outer-Level Electrons and Bonding Not all _____ in an atom take part in a bonding. Most bonds form using the outer most electrons called _________. - an electron in the outermost energy level of an atom.

To bond or Not to Bond Not all atoms bond the same way. The number of _______determines if an atom will form a bond. Atoms want to be like ______ (have a full outer shell of electrons). __ valence electrons like He Atoms bond by ________, or _____ electrons to have a ____ outer most energy level like the noble gases.

Ionic Bonds -are formed when _____ are _____ from one atom to another. Ionic bonds form so that the outermost energy levels of the atoms in the bonds are ______. Ionic bonds form between a ____ and a _______ Sodium Chlorine

Ionic Bonds Charged Particles (Magnets) A transfer of electrons between atoms changes the number of _____ in each atom but the number of ____ stay the _______. The negative charges and positive charges no longer _____ and the atom becomes an _______. _____- an atom that gained or lost electrons

Ionic Bonds Charged Particles Continued An atom cannot ____ electrons without a nearby atom _____ electrons and visa versa. Metals – tend to ______ electrons and become a ____ ion (_____) Nonmetals – tend to ______ electrons and become a ______ ion (____) _______ - the ending that is given to atoms that gain electrons Oxygen = _____ Fluorine = _____ Sulfur = _____

Ionic Compounds Metals tend to ______ a certain number of ____ depending on their number of _____ and become _______ charged Nonmetals tend to ______ a certain number of ____ depending on their number of _____ and become _______ charged
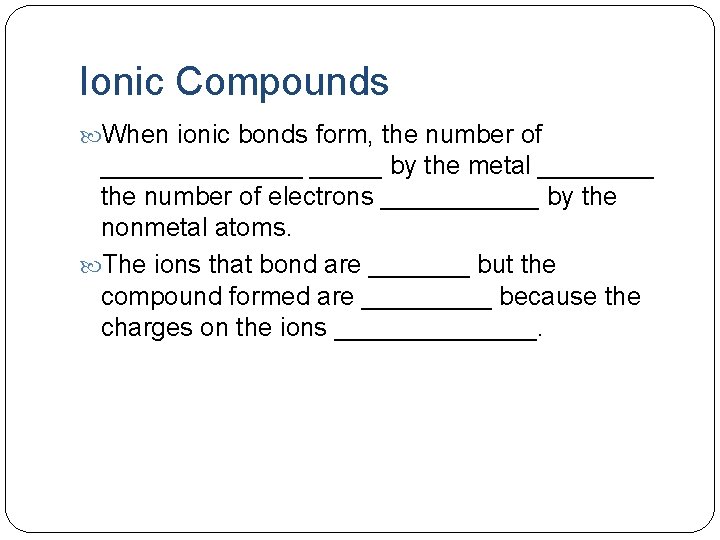
Ionic Compounds When ionic bonds form, the number of _______ by the metal ____ the number of electrons ______ by the nonmetal atoms. The ions that bond are _______ but the compound formed are _____ because the charges on the ions _______.

Ionic Compounds Writing Ionic Compounds ( _______ + _____) Write the metal with its’ charge Write the nonmetal with its’ charge Criss- Cross the charges Only bring down the #, NOT the charge Examples: Sodium and Chlorine Barium and Chlorine Potassium and Oxygen Litium and Sulfer

Ionic Compounds Naming Ionic Compounds Name of Metal + nonmetal w/ ending –ide Examples: Sodium and Chlorine Barium and Fluorine Potassium and Oxygen Lithium and Sulfur

Ionic Compounds When ions bond, they form a repeating 3 - dimensional pattern called a ________.

Covalent Compounds Covalent Bonds are formed when two ________ electrons. _______ – a bond that forms when atoms share one or more pairs of electrons

Covalent Compounds Examples of covalent compounds: 1. 2. 3. Properties 1. ____ melting points 2. ____ boiling points 3. Brittle as a solid Substances containing covalent bonds consist of individual particles called _________. _______ - a neutral group of atoms that are joined together by one or more covalent bonds.

Covalent Compounds Simple Covalent Compounds When found in nature as pure elements, these seven elements exist only as two atoms covalently bonded. H 2 O 2 7 N 8 O N 2 Cl 2 Br 2 I 2 F 2 1 H 9 F 19 Cl 35 Br 53 I

Covalent Compounds How to draw covalent molecules: using electron dot diagrams 1. Write the symbol of the element and place one dot around the symbol for every valence electron in the atom. Examples: Carbon Oxygen Krypton Hydrogen

Covalent Compounds 2. Combine the elements where they can share electrons so each have a full outer shell. (The shared electrons are the covalent bond) Examples: Hydrogen + Chlorine Fluorine + Fluorine Water Oxygen + Oxygen

Covalent Compounds Naming Covalent Compounds Rules: Prefixes One = _____ Two = _____ Three = _____ Four = _____ Five = _____ Six = _____ Seven = _____ Eight= _____ Nine = _____ Ten = _____ Prefix + name –ide Rule: Do NOT use mono- on the first element Examples: CO 2 C 2 H 4 P 2 O 5 N 3 P 6

Metallic Bonds Properties of Metals 1. 2. 3. Metals are malleable and ductile because of the presence of a __________________. Metallic Bond –

Metallic Bonds The _______ ions in a metal form a lattice that is held in place by strong _______ between the _____ ions and the surrounding _____. Swimming in electrons.
- Slides: 22











































