Energy Conservation Transfer Conductors and Insulators Lesson Essential












































- Slides: 46

Energy: Conservation & Transfer: Conductors and Insulators

Lesson Essential Questions How is thermal energy transferred? 2. Why do materials expand contract? 3. What materials are good conductors? 4. What materials are good insulators? Key Vocabulary thermal expansion, conductors, insulators, , heat, kinetic energy, temperature, volume (matter) 1.

Thermal Expansion and Contraction � When you apply a flame to the lid of a sealed food jar, the heat of the flame will loosen the lid's molecules, causing the lid to expand making it easier to open. When the lid cools down, however, its molecules will contract, causing the lid to shrink back to its normal size. � In this lesson, you will learn: � How an object's temperature causes it to expand or contract. � What is a good thermal/electrical conductor and what is a good thermal/electrical insulator.

Vocabulary Review � Heat - the transfer of thermal energy ◦ What happens to your hot soup if you take too long to eat it? ◦ It loses heat. ◦ Heat is what happens when thermal energy is gained or lost. ◦ To warm your soup up, you can add more energy using a microwave or stove. � Kinetic energy - the energy an object has because of its motion.

�Temperature -a measure of the average kinetic energy (energy of motion) of the atoms in a system, � Temperature is used to express thermal energy in degrees ◦ Temperature is the measure of how hot or cold something is. ◦ In physics, temperature is directly proportional to the amount of kinetic energy of the molecules of a substance. �If kinetic energy increases – temperature increases! �If kinetic energy decreases – temperature decreases! ◦ Temperature is measured in Kelvin, degrees Celsius or degrees Fahrenheit.

�Thermal of heat. energy - energy in the form ◦ Heat is the flow of energy from a hot object to a cooler one. ◦ When you feel that heat, in a cup of hot chocolate or the warmth of sunlight, you are actually feeling thermal energy. ◦ Thermal energy is the movement of molecules that make up the object. An object has more thermal energy when it is warm than when it is cool. ◦ Heat is the transfer of thermal energy from one object to another.

Heat and Temperature are NOT the same! � Heat is energy in motion. � Temperature is a measure of that energy. � http: //app. discoveryeducation. com/player/? a sset. Guid=E 45 E 9 DFA-8 C 07 -42 BA-AA 44438 D 7 AB 2506 F&layout=standalone

That’s hot! �Many things give off heat. �All heat is energy. Sun fire electricity

Campfire! � Pretend you are at a campfire. You are roasting a hot dog on a metal wire coat hanger. � Slowly the end of the hanger warms up, as it takes in the heat from the fire.

Ouch! � If you leave the hanger in the fire for a long time, the whole hanger will become to hot to touch! • Why does this happen?

Here’s the reason…. � This happens even though the handle end was never placed into the flames. � Heat moves from the flames to the metal handle. � From there it moves to all the molecules in the hanger by conduction until it reached your fingers.

Heat on the move! �Heat can move from one object to another, or from one molecule to another through the process of conduction. (RECALL LESSON #1)

Hot All Over! � As one molecule is heated it begins to move quickly. � When it does it passes some of its heat energy to other molecules around it. � When this happens, all the molecules of an object move heat from one to another, until they are all hot.

Heat Transfer � Heat can be transferred from one object to another through conduction, convection, or radiation. � http: //app. discoveryeducation. com/player/? a sset. Guid=1 EF 5319 F-966 E-4 A 38 -939 AA 66 BD 7 D 91 F 4 F&layout=standalone

Basics of HEAT FLOW ü HEAT - Always flows from regions of HIGHER temperature to regions of LOWER temperature. (RECALL – Lesson 1) ü HEAT - Always caused by a difference of temperature. ü HEAT - is the exciting of molecules.

How is heat conducted through solids, liquids, and gases? � Heat is conducted by the direct transfer of kinetic energy (energy of motion) from one object or substance to another. � Molecules transfer kinetic energy as they collide. � Objects or substances need to be in direct contact in order for conduction to occur.


�REVIEW!!!! � Heat is the transfer of thermal energy from a warmer object to a cooler object. � Temperature is a measure of the average kinetic energy of the particles that make up an object.

Remember…. Heat moves through objects. This is called conduction!

What things get hot fast? � Some materials (objects) get warmer faster than others. � Metal is a good conductor because it takes heat in quickly. � Copper becomes hot quickly, so it is a conductor. � A good conductor transfers heat quickly Copper pots

What are Conductors? � Conductors are materials that allow heat to move easily through them.

� Do you like toast? Did you ever look inside a toaster while it’s toasting bread? When you push down the lever to turn on the toaster, the metal heating element inside starts to glow orange or red almost instantly. You can see the glowing heating element inside this yellow toaster. � The glowing metal shows that the heating element has become very hot. It gets hot so quickly because metals are good conductors of thermal energy.

Good Conductors Many metals, like silver, copper, gold and aluminum, are good thermal conductors.

Conductors of Heat! Glass? ? ? Glass is a poor conductor of heat, especially in comparison to other materials, such as metal. Glass does conduct some heat, but does so more slowly than other materials.

You’re no good! �Other things like wood, plastics, and cotton are NOT good conductors of heat energy. �These are called insulators.

What are Insulators? � Insulators are materials that do not allow heat to move easily through them.

The trapped air acts as an insulator!!!

Feathers act as an insulator!

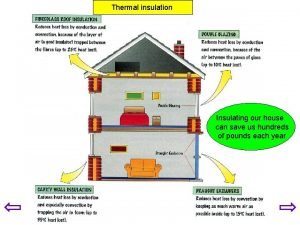
Good Thermal Insulators � Wood � Paper � Cotton � Wool � Plastic � Feathers � Dry air � Fiberglass

Thermal Insulator - AIR? ? ? ? � Air, like other gases, is a poor conductor of thermal energy. � The particles of gases are relatively far apart, so they don’t bump into each other or into other things as often as the more closely spaced particles of liquids or solids. � Therefore, particles of gases have fewer opportunities to transfer thermal energy. � Remember - Materials that are poor thermal conductors are called thermal insulators. �.

What do thermal Insulators help do?

What is an electrical conductor? � An electrical conductor is something which allows electricity to flow through easily. � An ◦ ◦ ◦ example of a conductor is: Scissors Paper clip Aluminum foil Iron Steel Copper

What is an electrical Insulator? � An electrical insulator does not allow electricity to flow through it easily. � Examples ◦ ◦ ◦ Cotton Paper Plastic Glass Rubber of insulators are:

These are pictures of conductors and Insulators Conductors Insulators

DEMONSTRATION Draw the following picture in your guided notes.

TAKE A LOOK! Set up the equipment as shown and tape the wires to the battery. • Does anything happen? • Does the banana allow electricity to flow through the circuit and light the bulb? • Is it a conductor or an insulator? Why or why not?

Inquiring Minds Want to Know! Conductor or Insulator? That is the question! Now lets look at the following materials! 1. Guess whether each item is a conductor or an insulator with a “X” � 2. As we test each item, record whether it was a conductor or and insulator with a Item Spoon Paper clip pencil Rubber band eraser Foil Nail coin Conductor Insulator Did the bulb light?

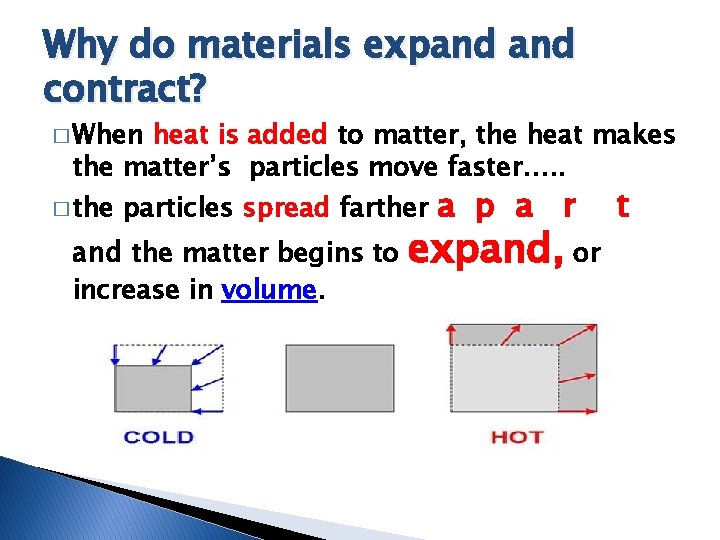
Why do materials expand contract? � When heat is added to matter, the heat makes the matter’s particles move faster…. . � the particles spread farther a p a and the matter begins to increase in volume. r expand, or t

� Removing heat causes the opposite process, and the matter contracts. � When an object cools it contracts.

� Different types of matter expand differently � SOLIDS - As a solid is heated the molecules vibrate more violently and the solid expands in all directions. � Different materials expand by different amounts for the same rise in temperature. � GASES - Under equal pressures, all gases expand at the same rate. � LIQUIDS - Unlike gases, liquids expand at different rates, depending on their composition. ◦ Liquids also expand by different amounts at different temperatures

Thermometers! � What causes the liquid in a thermometer to rise and fall? � The way a thermometer works is an example of heating and cooling a liquid. ◦ (THERMAL EXPANSION AND CONTRACTION) � When heated, the molecules of the liquid in thermometer move faster, causing them to get a little further apart. This results in movement up thermometer. � When cooled, the molecules of the liquid in thermometer move slower, causing them to get a little closer together. This results in movement down thermometer.

Solids vary greatly in their response to heat. Expansion joints are placed in roads, bridges, railroad tracks and sidewalks to help with expansion and contraction in these solids!

� On a hot day if a bridge, sidewalk, railroad track or road is NOT engineered to withstand thermal expansion and contraction…it could buckle!

Although most substances expand when heated, not all expand at the same rate. � Aluminum, for example, expands twice as much as iron when both are heated the same amount. � Rubber and water are two common substances that differ from most others in their response to heat. � Rubber contracts when heated instead of expanding! � Water also expands when it freezes!!!!! � The fact that water expands upon freezing causes icebergs to float. � Water expands when its temperature rises above (39° F. ) � Reference https: //www. physicsforums. com/threads/warmwater-expands. 117443/ � http: //app. discoveryeducation. com/player/? asset. Guid=B 2 DDCA 57 -FD 74 -4 DF 7 -A 0 FF 2 FF 76 EF 67002&layout=standalone

REVIEW - Why do materials expand contract? � An object’s temperature increases as a result of the kinetic energy of its molecules increasing…. . As an object’s molecules move faster and over greater distances, they take up more space, and the object expands. � An object cools as a result of the kinetic energy of its molecules decreasing. …. . As an object’s molecules move slower and over shorter distances, they take up less space, and the object contracts. � Different materials expand contract at different rates as heat energy is added or removed.

Review – Insulators & Conductors �Insulator- does not allow heat/electricity to travel through it easily. �Conductor- does allow heat/electricty to travel through it easily. �REVIEW QUIZ/GAME �http: //www. ck 12. org/book/CK-12 Physical-Science. Concepts/section/5. 17/ �CLICK ON PRACTICE TAB – COMPLETE QUIZ/GAME
 Conductor examples
Conductor examples Is scissors a conductor or insulator
Is scissors a conductor or insulator Calculate voltage across resistor
Calculate voltage across resistor Table of conductors and insulators
Table of conductors and insulators Bad conductor of electricity
Bad conductor of electricity Studyjams
Studyjams Physics 2135
Physics 2135 Penny insulator or conductor
Penny insulator or conductor Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Transfer heat
Transfer heat Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Plamatic acid
Plamatic acid Lesson 2 energy transfer in the atmosphere
Lesson 2 energy transfer in the atmosphere Opacity and translucency in insulators
Opacity and translucency in insulators Chapter 11 study guide energy and its conservation answers
Chapter 11 study guide energy and its conservation answers Fluid
Fluid Chapter 11 energy and its conservation answers
Chapter 11 energy and its conservation answers Energy conversion and conservation
Energy conversion and conservation Conservation of mass momentum and energy equations
Conservation of mass momentum and energy equations Single transformation of energy
Single transformation of energy Pole vault energy transformation
Pole vault energy transformation Energy meaning in science
Energy meaning in science Initial potential energy
Initial potential energy A cannon sits on a stationary railroad flatcar
A cannon sits on a stationary railroad flatcar Energy conversion of a toaster
Energy conversion of a toaster Insulators example
Insulators example Insulating house
Insulating house Bad insulators
Bad insulators Heat conductors
Heat conductors Victor insulators inc
Victor insulators inc How does a rubber rod become negatively
How does a rubber rod become negatively Testing of insulators
Testing of insulators Wave is a disturbance that transfers energy
Wave is a disturbance that transfers energy Equation for kinetic energy
Equation for kinetic energy Change in energy quick check
Change in energy quick check Derive kinetic energy formula
Derive kinetic energy formula Explain the law of conservation of mechanical energy
Explain the law of conservation of mechanical energy Law of conservation of energy worksheets
Law of conservation of energy worksheets Conservation of kinetic energy formula
Conservation of kinetic energy formula Conservation of enthalpy
Conservation of enthalpy First law of td
First law of td Boiler efficiency direct method
Boiler efficiency direct method Mechanical energy
Mechanical energy Energy conservation law
Energy conservation law Conservation of energy youtube
Conservation of energy youtube Law of conservation of energy example
Law of conservation of energy example