Ideal and Dilute Solutions Master Thermodynamics Equations Chemical

























- Slides: 28

Ideal and Dilute Solutions

Master Thermodynamics Equations

Chemical Potential Diffusion from high to low potential. Chemical potential is a Partial Molar Quantity Sum of moles of components

Chemical Potential of a Binary (A & B) Mixture Chem. Potential applied to other variables:

Other Partial Molar Quantities Partial Molar Volume: Partial Molar Enthalpy: Partial Molar Entropy:

Calculation for Partial Molar Volumes V = f(n. A , n. B) @ constant P & T

Raoult’s Law & Ideal Solutions Vapor Pressure (VP) Pi (escaping tendency g) Gas Ideality => No Intermolecular forces Solution Ideality => Uniformity in Intermolecular forces. (Binary: A-A , B-B , A-B all the same) Dalton’s Law

Raoult’s Law & Ideal Solutions

Thermodynamics of Mixing for an Ideal Solution

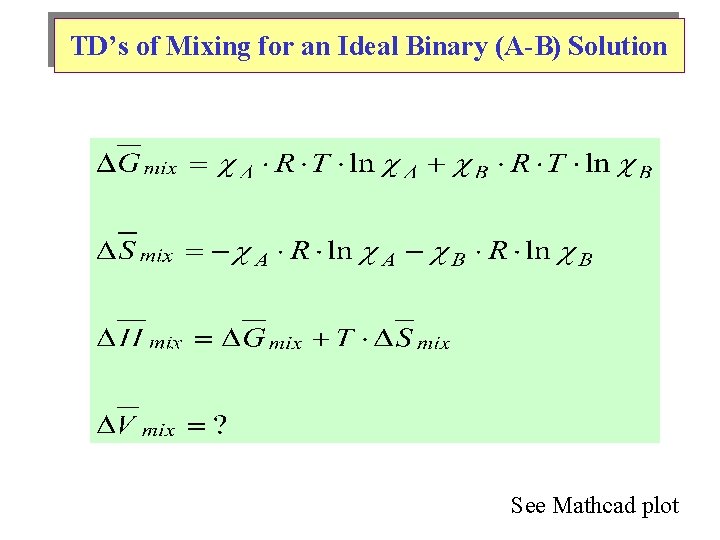
TD’s of Mixing for an Ideal Binary (A-B) Solution See Mathcad plot


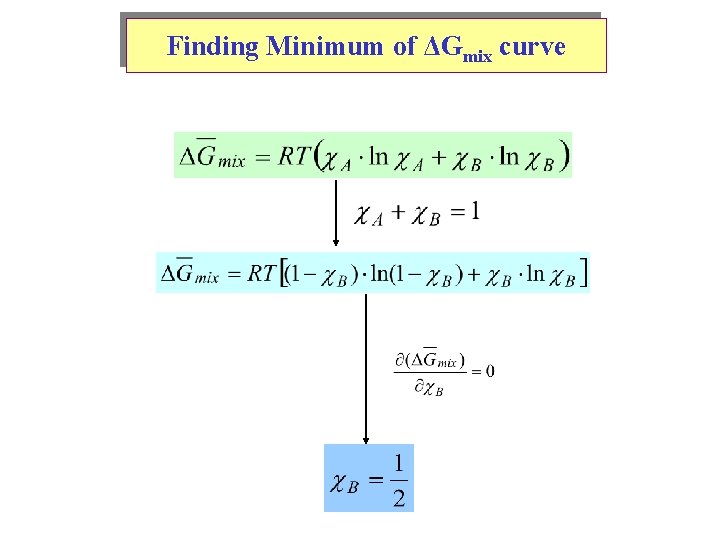
Finding Minimum of ΔGmix curve

Henry’s Law (Solubility of gases in liquids) In dilution solutions, each solute is surrounded by solvent molecules (uniform environment, relatively ‘ideal. ’) Positive and Negative deviations from Raoult’s Law Endothermic Mixing versus Exothermic Mixing


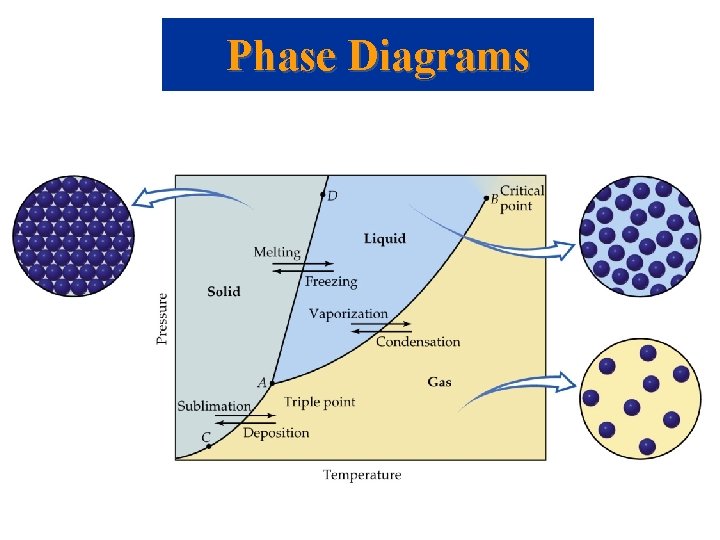
Phase Diagrams

Phase Diagrams The Phase Diagrams of H 2 O and CO 2

Phase Diagrams for Multi-components For 2 components: Need 3 variables ( T , P , composition ) P Most common plots: T VP vs. @ constant T B. pt. vs. @ constant P

Phase Diagrams for Multi-components Liquidus Curve: Vapour Curve:

Phase Diagrams for Multi-components


Colligative Properties Boiling-Point Elevation • Molal boiling-point-elevation constant, Kb, expresses how much Tb changes with molality, m. S: • Decrease in freezing point ( Tf) is directly proportional to molality (Kf is the molal freezing-point-depression constant):

Figure 13. 22

Solubility ( Conc’n vs. T ) Derivation starting with equilibrium thermodynamics, At equilibrium (constant P & T):

Freezing Point Depression ( T vs. conc’n ) Kf = molal freezing point constant, all properties of the solvent A [ units = K kg mol-1 ] Similar equation for Tb

Colligative Properties Osmosis • movement of a solvent from low solute concentration to high solute concentration across a semipermeable membrane. Figure 13. 23

Colligative Properties Osmosis • Osmotic pressure, , is the pressure required to stop osmosis:

Application to Polymeric Solutions

Ideal and Dilute Solutions
 Dilute and concentrated solutions
Dilute and concentrated solutions Dilute and concentrated solutions
Dilute and concentrated solutions Are kc and kp equal
Are kc and kp equal Translating chemical equations
Translating chemical equations Concentrated weak acid
Concentrated weak acid Common ion solubility
Common ion solubility Types of desizing methods
Types of desizing methods Dilute solution definition
Dilute solution definition Flocculated and deflocculated suspension
Flocculated and deflocculated suspension Which solution
Which solution Ideal solution thermodynamics
Ideal solution thermodynamics First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Dr. erukhimova
Dr. erukhimova Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Equilibrium thermodynamics
Equilibrium thermodynamics Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Thermodynamics chapter 2
Thermodynamics chapter 2 Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 熱力
熱力 Thermodynamics equations
Thermodynamics equations Gibbs helmholtz equation
Gibbs helmholtz equation Partial vapour pressure
Partial vapour pressure Chemical and petroleum solutions
Chemical and petroleum solutions Equations and their solutions
Equations and their solutions Equations and their solutions
Equations and their solutions Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Formula of love
Formula of love