Theory of Atomic Structure Greeks Democritus Leucippus n






























- Slides: 32

Theory of Atomic Structure

Greeks – Democritus, Leucippus n n n Over 2000 years ago All matter is composed of tiny particles These particles are so small that they cannot be broken down (indestructible) Named these particles atoms (from the Greek work for indestructible) Common Greek theory was that all matter consisted of four "elements" - earth, air, fire, and water

John Dalton n 1803 - Proposed an "atomic theory" with spherical solid atoms based upon measurable properties of mass n n n All elements are composed of atoms, which are indivisible and indestructible particles All atoms of the same element are exactly alike; in particular they all have the same mass Joining atoms of two or more elements forms compounds. In any compound, the atoms are joined in a definite whole number ratio (H 2 O, 2 to 1 ratio)

Dalton n Did account for the following laws: n n Law of conservation of mass (a chemical change is simply a rearrangement of atoms) Law of definite proportions (atoms are combined in definite ratios) Law of multiple proportions (elements can combine in different ratios to form new compounds – H 2 O, H 2 O 2 Did not account for isotopes

J. J Thompson n Through the use of cathode rays, Thompson was able to discover a negatively charged particle with almost no mass n Discovered n electrons See Cathode Ray Tube

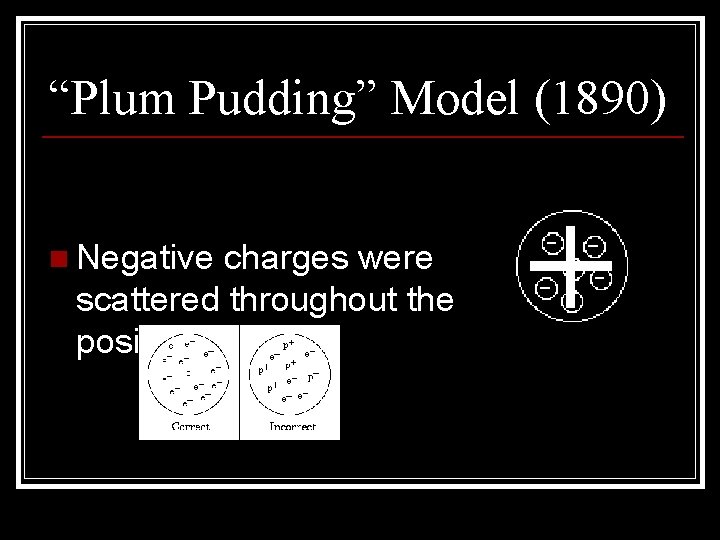
“Plum Pudding” Model (1890) n Negative charges were scattered throughout the positive atom

Ernest Rutherford n n Discovered that atoms have a positive, dense nucleus surrounded by negative “empty” space (the electrons are in the “empty” space) Gold Foil Experiment – 1909 (chem ASAP)

Gold Foil Experiment n What n Alpha (α) particles – positively charged n n Same as Helium nuclei (42 He 2+) Gold Foil – only a few atoms thick n What n was used was done Shot a beam of α particles through the gold foil

Gold Foil Experiment n What happened? The majority of the particles went straight through the foil and exposed the film behind it n A few particles were deflected back from the foil and scattered n

Gold Foil Experiment n What does this mean? n Since the α particles are (+) charged, they must have been pulled through the foil by a strong (-) charge n n The atom was mostly negatively charged “empty” space Since some α particles bounced back, they must have hit a positive part of the foil n The atom must have a small positive part located in the center

Gold Foil Experiment n Atomic Theory (Conclusion) n n An atom consists of a very small, positively charged nucleus and the rest is negatively charged empty space. This empty space must contain the electrons. Lets see the experiment again!

Bohr Model – Neils Bohr 1919 Electrons resemble our solar system n Electrons revolve (orbit) around the nucleus n Principle energy levels (PEL) = rings around the nucleus n

Principle Energy Levels n n The only regions in which electrons can be found Each level can only contain a specific number of electrons (2 in the 1 st, 8 in the 2 nd) Electrons farther away from the nucleus have higher energy Number of PEL’s is given by the period number (found on the periodic table)

James Chadwick 1932 – discovered neutrons n Using alpha particles he discovered a neutral atomic particle with a mass close to a proton. n

Valence Electrons – outer electrons Outermost electrons give an atom its properties n These are the electrons that are involved in reactions n Last number in the electron configuration n Kernel electrons – inner electrons n

Electron Dot Symbols / Lewis Dot Diagrams n n 1. 2. 3. 4. 5. 6. Dots represent valence electrons Examples: K Ca S Ar Ca 2+ S 2 -

Electron Configurations Distribution of electrons n Given in the lower left hand corner of the periodic table (below the atomic number) n

Rules 1. 2. 3. 4. 5. 1 st PEL can hold 2 electrons 2 nd PEL can hold 8 electrons 3 rd PEL can hold 18 electrons 4 th PEL can hold 32 electrons No more than 8 electrons are in the outermost principle energy level n 6. This equal 8 valence electrons, the maximum amount of valence electrons possible The electrons are added one at a time to the unfilled principle energy levels

Examples n Ca: 2 -8 -8 -2 What does this mean? n n 4 principle energy levels contain electrons 1 st PEL contains 2 e-, 2 nd and 3 rd PEL contain 8 e -, and the 4 th PEL contains 2 e 1 st and 2 nd are filled, 3 rd and 4 th are not completely full Ca has 2 valence electrons, and 18 kernel electrons

Examples: 1. C 2. Li 3. F

Detailed Electron Configurations n Within each principle energy levels there are sublevels n Each s sublevel contains 2 electrons n Each p sublevel contains 6 electrons n Each d sublevel contains 10 electrons n Each f sublevel contains 14 electrons

PEL Sublevels # of electrons 1 s 2 2 s, p 2+6=8 3 s, p, d 2 + 6 + 10 = 18 4 s, p, d, f 2 + 6 + 10 + 14 = 32

Example n Calcium: n n 1 s 22 p 63 s 23 p 64 s 2 What does this mean? ? ? n n The number in front of the sublevel indicates the PEL (4 s = 4 th PEL, s sublevel) The number of electrons is given by the superscript (4 s 2 = 4 th PEL, s sublevel, 2 e-)

Rules n Fill the lowest energy sublevels first n n See energy chart and diagonal method No more than 8 electrons can be in the outermost principle energy level

Examples 1. C 2. Al 3. Ca 4. Ni

Ground State Electrons are in the lowest available energy levels n This is how the electron configuration is written on the periodic table n Normal configuration n Stable n

Excited State When an electron gains energy, the electron is at a higher energy state (excited state) n Electrons absorb energy and move to new higher energy levels n UNSTABLE n

Excited State n n n When an electron returns from the excited state to the ground state, energy is released The energy is often emitted in the form of light The wavelength (color) of the light can be used to identify the element because each element absorbs and releases a specific amount of energy

Quantum Theory n n Electrons can only absorb (or release) specific amounts of energy called quanta This energy corresponds to the differences between energy levels Energy is always absorbed (or released) in definite amounts rather than in a continuous flow A quantum of radiation is called a photon

Flame Tests Every element absorbs and hence emits different amounts of energy (different colors) n Can be used for identification n

Spectra/Spectroscope • Spectra – The photons of light that are given off can be broken down into lines (spectral lines) • Spectroscope – An instrument that breaks light into colored bands – Every element has a unique band length (arrangement)

Modern Orbital Theory (Wave Mechanical/Electron Cloud Model) Electrons do not have a specific path or location – they have probabilities of being located there n Regions of high probabilities are called orbitals n n Electrons are located in orbitals
 When did leucippus discover the atomic theory
When did leucippus discover the atomic theory Democritus theory of the universe
Democritus theory of the universe Democritus atomic theory
Democritus atomic theory Bc atomic mass
Bc atomic mass Democritus atomic theory
Democritus atomic theory Atomic theory timeline democritus
Atomic theory timeline democritus Democritus atomic model diagram
Democritus atomic model diagram History of the atom scientists
History of the atom scientists Early greece map
Early greece map Which statement is accurate?
Which statement is accurate? Why did tyrants fall out of favor with the greeks
Why did tyrants fall out of favor with the greeks Geometric mass floral design
Geometric mass floral design Romans and greeks
Romans and greeks Early american period floral design
Early american period floral design Ancient greek cultural values
Ancient greek cultural values Above all else i must be saved
Above all else i must be saved Where does the word theatre come from
Where does the word theatre come from Geocentric model
Geocentric model Doci timeo danaos
Doci timeo danaos Draw segment sr the bisector of the vertex angle prq
Draw segment sr the bisector of the vertex angle prq Option greeks wikipedia
Option greeks wikipedia Relative formula mass of hcl
Relative formula mass of hcl Trends of periodic table
Trends of periodic table Atomic size periodic trend
Atomic size periodic trend Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Distinguish between mass number and atomic mass.
Distinguish between mass number and atomic mass. Atomic number vs atomic radius
Atomic number vs atomic radius Demokritos atom modeli
Demokritos atom modeli Democritus atom model
Democritus atom model Democritus contribution
Democritus contribution Aristotle democritus
Aristotle democritus Perkembangan model atom rutherford
Perkembangan model atom rutherford Nuclear symbol notation
Nuclear symbol notation