Abstract
Introduction: Working memory (WM) and its blood-oxygen-level-dependent-related parametric modulation under load decrease with age. Functional connectivity (FC) generally increases with WM load; however, how aging impacts connectivity and whether this is load-dependent, region-dependent, or associated with cognitive performance is unclear.
Methods: This study examines these questions in 170 healthy adults (meanage = 52.99 ± 19.18) who completed functional magnetic resonance imaging scanning during an n-back task (0-, 2-, 3-, and 4-back). The FC was estimated by utilizing a modified generalized psychophysiological interaction approach with seeds from fronto-parietal (FP) and default mode (DM) regions that modulated to n-back difficulty. The FC analyses focused on both connectivity during WM engagement (task vs. control) and connectivity in response to increased WM load (linear slope across conditions). Each analysis utilized within- and between-region FC, predicted by age (linear or quadratic), and its associations with in- and out-of-scanner task performance.
Results: Engaging in WM either generally (task vs. control) or as a function of difficulty strengthened integration within- and between-FP and DM regions. Notably, these task-sensitive functional connections were robust to the effects of age. Stronger negative FC between FP and DM regions was also associated with better WM performance in an age-dependent manner, occurring selectively in middle-aged and older adults.
Discussion: These results suggest that FC is critical for engaging in cognitively demanding tasks, and its lack of sensitivity to healthy aging may provide a means to maintain cognition across the adult lifespan. Thus, this study highlights the contribution of maintenance in brain function to support working memory processing with aging.
Impact statement
The literature examining functional connectivity (FC) during working memory (WM) in healthy adults is mixed in both age effects and its relationship to performance. This study contributes to the literature by examining a large, adult lifespan sample, increased levels of WM load, and additional investigation of connections within and between fronto-parietal and default mode regions. Results revealed age-invariant strengthened FC during WM, suggesting that healthy aging may be resilient to FC changes. In addition, negative FC between regions was associated with better WM performance in middle-aged and older adults, highlighting the importance of FC maintenance to support successful WM ability.
Keywords: aging, cognitive control, functional connectivity, functional integration, n-back, psychophysiological interactions
Introduction
Working memory (WM) ability, generally defined as the cognitive process that involves temporarily storing and manipulating information (Baddeley and Hitch, 1974; Baddeley, 2000), decreases with age (Artuso et al., 2017; Dobbs and Rule, 1989; Dumas et al., 2001; Park et al., 2002). WM relies on a network of regions bilaterally in the frontal (e.g., lateral and medial prefrontal cortex and frontal operculum) and parietal lobes (e.g., intraparietal sulcus) (Owen et al., 2005; Rottschy et al., 2012); however, the way in which these brain regions support WM across the adult lifespan is poorly understood.
Functional magnetic resonance imaging (fMRI) methods allow for the estimation of brain activation or connectivity under varying WM demand. Activation studies provide insight into the differential activation of brain regions during cognitive performance, whereas connectivity studies indicate how the brain synchronizes activity over time. Each method provides unique, but complementary, information about how the brain supports WM and maintains WM performance over time. Activation studies often report robust age differences in brain activation as WM demand increases; however, task-based connectivity studies are more mixed in the reporting of age effects, especially as related to supporting cognition.
Brain activation has been shown to modulate as a function of WM load increase (Kennedy et al., 2017; Owen et al., 2005; Rottschy et al., 2012; Wager and Smith, 2003). In addition, both positive modulation (i.e., increasing activity) of fronto-parietal (FP) regions and negative modulation (i.e., decreasing activity) of default mode (DM) regions to increasing WM load are reduced with increasing age (e.g., Kennedy et al., 2017), which is in accord with the default-executive coupling hypothesis of aging (DECHA) model (Turner and Spreng, 2015). The DECHA posits that the coupling of activation in the fronto-parietal network (FPN) regions alongside suppression of default-mode network (DMN) regions during executive function tasks, including WM, is altered with aging, possibly resulting in poorer cognitive performance.
Maintaining synchronous activity over time (e.g., functional integration) is also critical to supporting complex cognitive functions. Functional integration of brain regions during a given cognitive task is necessary for proper task performance, and improper coupling of regions or networks likely underlies individual differences in performance (Shine et al., 2016). Functional connectivity (FC), a proxy for functional integration, has been explored during WM and demonstrates increases both within and between FP and DM regions under greater WM load (Di and Biswal, 2018; Hakun et al., 2015; Heinzel et al., 2014, 2017; Nagel et al., 2011; Newton et al., 2011). The sensitivity of connectivity to task difficulty suggests that FC is an essential brain function that supports complex cognition (Smith et al., 2016). Thus, it is critical to understand FC differences across the adult lifespan and further, how this brain property relates to cognitive performance.
Despite the consistent finding that connectivity in the FPN and DMN are responsive to task demands, age group comparison studies of younger versus older adults have revealed differential patterns of FP connectivity to increasing WM load (Hakun et al., 2015; Heinzel et al., 2014, 2017; Honey et al., 2002; Nagel et al., 2011). For instance, during the n-back paradigm across studies, older adults evidence decreased connectivity in FP regions during the 3-back condition (Heinzel et al., 2014, 2017), decreased FC for 3-back compared with 1-back conditions (Heinzel et al., 2014; Nagel et al., 2011), and also increased connectivity during 2-back condition (Heinzel et al., 2014). Similarly, the few studies that examine changes in FC to increasing task difficulty between FP-DM regions with regard to aging are equivocal, reporting both weakened negative FC (anti-phase synchronization) and reversed FC (strengthened in-phase synchronization) with aging (Hakun et al., 2015; Turner and Spreng, 2015).
In addition to these FC findings, we recently reported that the significant inverse correlation between positive modulation clusters (which included largely FP regions) and negative modulation clusters (which included largely DM regions) to increased WM load was invariant to aging (Kennedy et al., 2017). We interpreted this functional coupling (i.e., strengthened positive modulation coupled with weakened negative modulation), as an individual difference measure that gauged the correlated modulation of these FP and DM regions, as would be predicted by the DECHA framework (Turner and Spreng, 2015). Importantly, that coupling was derived from a correlation of modulated activation and is not a direct measure of FC. However, the finding provides indirect support for an age-invariant property of network coupling in healthy aging (e.g., between FP and DM regions).
Finally, an important component of understanding the broader significance of aging effects on task-based connectivity is evidence for reliable association between FC and cognitive performance. To date, the literature on connectivity among FP regions, age, and cognitive performance reports positive associations across the adult lifespan, positive associations in younger, but not older adults, as well as null findings (Heinzel et al., 2014, 2017; Nagel et al., 2011). Further, the impact of aging on between FP and DM coupling during WM with WM performance is unexamined. Thus, a gap exists in the understanding of this critical metric of brain function as it relates to aging and to cognitive performance.
Therefore, the current study examines FC (using modified generalized psychophysiological interactions; gPPI) both within- and between FP and DM regions that previously demonstrated parametrically modulated activation in response to working memory load during an n-back task (in Kennedy et al., 2017). We computed PPI models for two contrasts: WM task compared with control conditions (2-3-4 vs. 0-back), and increasing working memory load across conditions (2-3-4 back slope). For each of these contrasts, we sought to determine (1) the pattern of within- and between FP and DM region connectivity, (2) whether these within- and/or between-region connectivity patterns differ with age (linearly or non-linearly), and (3) whether within- and/or between-region connectivity was predictive of working memory performance (measured both inside and outside-of-scanner) across the adult lifespan. Both linear and non-linear age were modeled given the non-linear age effects previously reported in both working memory performance (Schneider-Garces et al., 2010; Swanson, 1999) and in resting-state FC (Cao et al., 2014; Ng et al., 2016).
Based on the limited literature, we expected (1) strengthened positive connectivity within-FP and within-DM regions (i.e., in-phase synchronization), and strengthened negative connectivity between FP and DM regions (i.e., anti-phase synchronization) when engaged in the task and as the task n-back load increased; and (2) given the limited and mixed associations of FC to aging and to cognitive performance, we suspected that FC could be age-invariant, but that its association to cognitive performance may be load- and/or age-dependent.
Materials and Methods
Participants
Participants consisted of 170 healthy adults aged 20–94 years (mean = 52.99, standard deviation = 19.18) recruited via media advertisements and flyers from the greater Dallas-Fort Worth metroplex, which is the same sample as described in Kennedy et al. (2017). Exclusion criteria included cardiovascular disease, diabetes, cognition-altering medication, and a history of head trauma with loss of consciousness >5 min, substance abuse, neurological, or psychiatric disorders. Participants were required to score >25 on the mini-mental state exam (MMSE; Folstein et al., 1975) and ≤16 on the Center for Epidemiological Study Depression Scale (CESD; Radloff, 1977). All participants were native English speakers, right-handed, had a minimum of high school education or equivalent, normal or corrected-to-normal vision, and provided informed consent according to the UT Southwestern Medical Center and UT Dallas institutional review boards. See Table 1 for participant demographics. Sex, years of education, and CESD did not significantly differ with age in this sample, but as is typical, MMSE and d′ both decreased with increasing age (MMSE: b = −0.012, t[168] = −3.53, p < 0.001, d′: b = −0.021, t[168] = −7.56, p < 0.001).
Table 1.
Participant Demographics by Age Group: Means (±Standard Deviation)
| Age group |
Total | ||||
|---|---|---|---|---|---|
| Younger (20–35) | Middle (36–55) | Older (56–69) | Oldest (70–94) | ||
| n | 42 | 47 | 37 | 44 | 170 |
| % women | 0.57 | 0.55 | 0.59 | 0.64 | 0.59 |
| Age | 27.69 (4.42) | 46.00 (5.65) | 61.84 (3.46) | 77.18 (6.17) | 52.99 (19.18) |
| Education (years) | 15.48 (2.21) | 15.28 (2.52) | 15.84 (2.37) | 15.66 (2.88) | 15.55 (2.50) |
| MMSE*** | 29.19 (0.94) | 29.28 (0.80) | 28.89 (0.77) | 28.77 (0.83) | 29.04 (0.86) |
| CESD | 4.48 (3.62) | 4.98 (4.48) | 3.27 (2.86) | 3.77 (3.73) | 4.17 (3.79) |
| d′*** | 2.35 (0.51) | 1.80 (0.92) | 1.59 (0.65) | 1.28 (0.54) | 1.75 (0.78) |
Participants are binned into younger, middle, older, and oldest adult age groups for descriptive purposes. Age was used as a continuous variable in all analyses. Mean and standard deviation (in parentheses) of age, years of education, MMSE, and d′ are reported for each group and overall sample. Sex, years of education, and CESD did not significantly differ with age. However, MMSE and d′ significantly decreased with increasing age.
p < 0.001.
CESD, Center for Epidemiological Study Depression; d′, discriminability index; MMSE, Mini-Mental State Examination.
Procedures
Participants completed two cognitive assessment sessions followed by an MRI session, each completed on separate days and lasting ∼2 h; see Kennedy et al. (2017) for an unabridged description. During the cognitive sessions, participants completed a battery of cognitive tests that examined cognitive processes such as executive function, memory, problem-solving, and reasoning. During the MRI session, participants were trained on the in-scanner task followed by a collection of multimodal MRI images, including functional and structural MR images.
Working memory (Wechsler Adult Intelligence Scale-digit span)
During the cognitive sessions, participants completed the digit span subtest of the Wechsler Adult Intelligence Scale (WAIS-DS) (Wechsler, 2008). The WAIS-DS consisted of a forward, backward, and sequencing subsection where participants were given a series of numbers and were instructed to list the numbers in the same order as given (forward), in reverse order as given (backwards), or in numerical order (sequencing). Given that the DS sequencing subtest was the most difficult task and most aligns with the n-back paradigm, this subtest was analyzed as a measure of out-of-scanner WM performance (Kennedy et al., 2017).
MRI acquisition
All participants were scanned on a single 3T Philips Achieva scanner equipped with a 32-channel head coil at the Advanced Imaging Research Center at the University of Texas Southwestern Medical Center. Functional data during an n-back task were collected by using a T2*-weighted echo-planar imaging sequence with 29 interleaved axial slices per volume providing full brain coverage and acquired parallel to the anterior commissure-posterior commissure line (64 × 64 × 29 matrix, 3.4 × 3.4 × 5 mm3, field of view = 220 mm2, echo time [TE] = 30 ms, repetition time [TR] = 1.5 sec, flip angle = 60°). High resolution structural images were also collected with a T1-weighted magnetic prepared rapid gradient echo sequence (160 sagittal slices, 1 × 1 × 1 mm3 voxel size; 256 × 204 × 160 matrix, TR = 8.3 msec, TE = 3.8 msec, flip angle = 12°).
n-back training
To ensure understanding of the n-back paradigm, participants were trained on the task before entering the scanner. Participants were first given instructions regarding the 0-back condition and were taught how to respond if a number was the same (using their index finger to press the left button) or different (using their middle finger to press the right button) than an instructed number using an identical button box to that used in-scanner. Participants practiced the 0-back until they achieved >80% accuracy. Participants were then trained similarly for the 2, 3, and 4-back conditions, with most participants achieving 80% accuracy on all conditions before entering the scanner. After practicing each WM load condition, participants completed an abbreviated version of the full task.
fMRI task: digit n-back
In the scanner, participants completed three functional runs of the n-back task in a block design. The task was presented, and behavior was recorded by using PsychoPy software v1.77.02 (Peirce, 2007, 2009). Participants viewed the stimuli on a monitor mounted to the rear of the scanner, which was visible via a mirror mounted on the head coil. For each run, two blocks of each condition were presented in a pseudo-counterbalanced order. Each 0-back block included 10 trials, whereas each 2, 3, and 4-back block included 20 trials. For each block, a 5-sec cue indicated which n-back load was to follow (i.e., 0-, 2-, 3-, or 4-back). The cue was followed by a 2-sec fixation cross before the presentation of the digit. Digits 2–9 were pseudo-randomly presented for 500-ms with a 2000-ms inter-stimulus interval. Of the 420 trials, 144 (34.3%) were match (same) trials (i.e., 18 [4.2%] for 0-back and 42 [10.0%] each for 2, 3, and 4-back) and 276 (65.7%) were non-match (different) trials (i.e., 42 [10.0%] for 0-back and 78 [18.6%] each for 2, 3, and 4-back).
fMRI preprocessing
Before preprocessing, each scan was visually inspected for quality and motion artifacts. A standard preprocessing pipeline using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) via Matlab R2012b (Mathworks) consisted of realignment, co-registration of functional to T1 anatomical images, warping functional images to Montreal Neurological Institute (MNI) space using the T1 anatomical to MNI warp, and spatial smoothing of the functional images using an 8 mm full-width half-maximum Gaussian kernel. Artifact Repair Toolbox was also utilized to examine movement and intensity shift in the functional images (Mazaika et al., 2005). Volumes were marked as outliers if movement was >2 mm of translation or 2° of rotation, or an intensity shift >3% deviation from the mean global intensity shift. Functional runs were excluded if 40 or more volumes (i.e., 15% of total volumes) were marked as outliers for movement. Only participants with at least two functional runs were included in the subsequent PPI and group analyses.
Seven participants were excluded from further analyses: more than one functional run marked as an outlier (n = 3), poor quality T1-weighted scan acquisition (n = 2), provided no response to >15% of the trials (n = 1), and failed preprocessing step for PPI analyses (n = 1).
Regions of interest
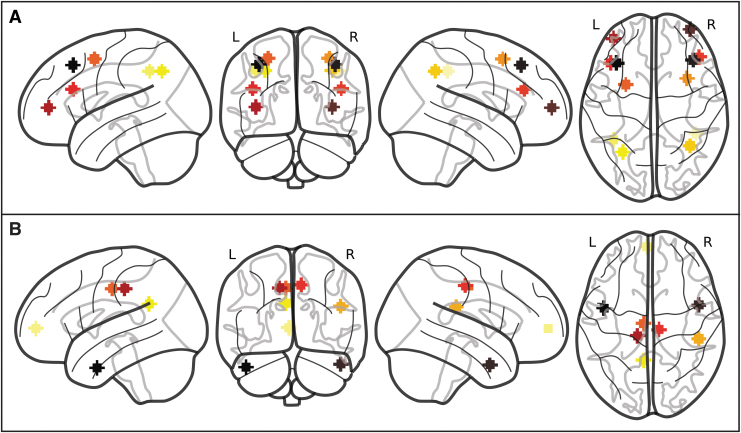
Seed regions of interest (ROIs) were chosen from the statistical map derived from the contrast of positive and negative modulation in response to n-back load as reported in table 2 and figure 2 of Kennedy et al. (2017). Given the large cluster sizes resulting from the task activation analysis, we extracted several additional peaks/subpeaks to inform the current FC analysis that are solely within FP and DM regions (Table 2 and Fig. 1). FP regions were chosen from peak global and local maxima from within clusters showing positive modulation. The DM regions were chosen from peak global and local maxima from within clusters demonstrating negative modulation. Spheres with 6 mm radius were created from each maxima coordinate. The seed ROIs were also used as target ROIs.
Table 2.
Regions of Interest: 6 mm-Radius Spheres Used as Both Seeds and Targets
| ROI | MNI |
Network | Hemisphere | Region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | 42 | −42 | 42 | Fronto-parietal | R | Supramarginal gyrus |
| 2 | −39 | −48 | 42 | Fronto-parietal | L | Inferior parietal lobule |
| 3 | −30 | −60 | 42 | Fronto-parietal | L | Inferior parietal lobule |
| 4 | 36 | −54 | 42 | Fronto-parietal | R | Inferior parietal lobule |
| 5 | 33 | 12 | 54 | Fronto-parietal | R | Middle frontal gyrus |
| 6 | −27 | 6 | 54 | Fronto-parietal | L | Middle frontal gyrus |
| 7 | 45 | 33 | 24 | Fronto-parietal | R | Inferior frontal triangularis |
| 8 | −42 | 27 | 24 | Fronto-parietal | L | Inferior frontal triangularis |
| 9 | −39 | 51 | 6 | Fronto-parietal | L | Middle frontal gyrus |
| 10 | 36 | 60 | 6 | Fronto-parietal | R | Middle frontal gyrus |
| 11 | 39 | 30 | 48 | Fronto-parietal | R | Middle frontal gyrus |
| 12 | −36 | 27 | 48 | Fronto-parietal | L | Middle frontal gyrus |
| 13 | −3 | 60 | 3 | Default | L | Medial superior frontal gyrus |
| 14 | −6 | −51 | 27 | Default | L | Posterior cingulum |
| 15 | 48 | −30 | 24 | Default | R | Supramarginal gyrus |
| 16 | −6 | −15 | 42 | Default | L | Middle cingulum |
| 17 | 9 | −21 | 45 | Default | R | Middle cingulum |
| 18 | −12 | −27 | 42 | Default | L | Middle cingulum |
| 19 | 48 | 3 | −33 | Default | R | Inferior temporal gyrus |
| 20 | −45 | 0 | −36 | Default | L | Inferior temporal gyrus |
These regions were chosen from peak global and local maxima from prior results of positive and negative parametric modulation of the n-back task within fronto-parietal and default mode regions (Kennedy et al., 2017). x, y, and z coordinates are in MNI atlas space.
L, left; MNI, Montreal Neurological Institute; R, right; ROI, region of interest.
FIG. 2.
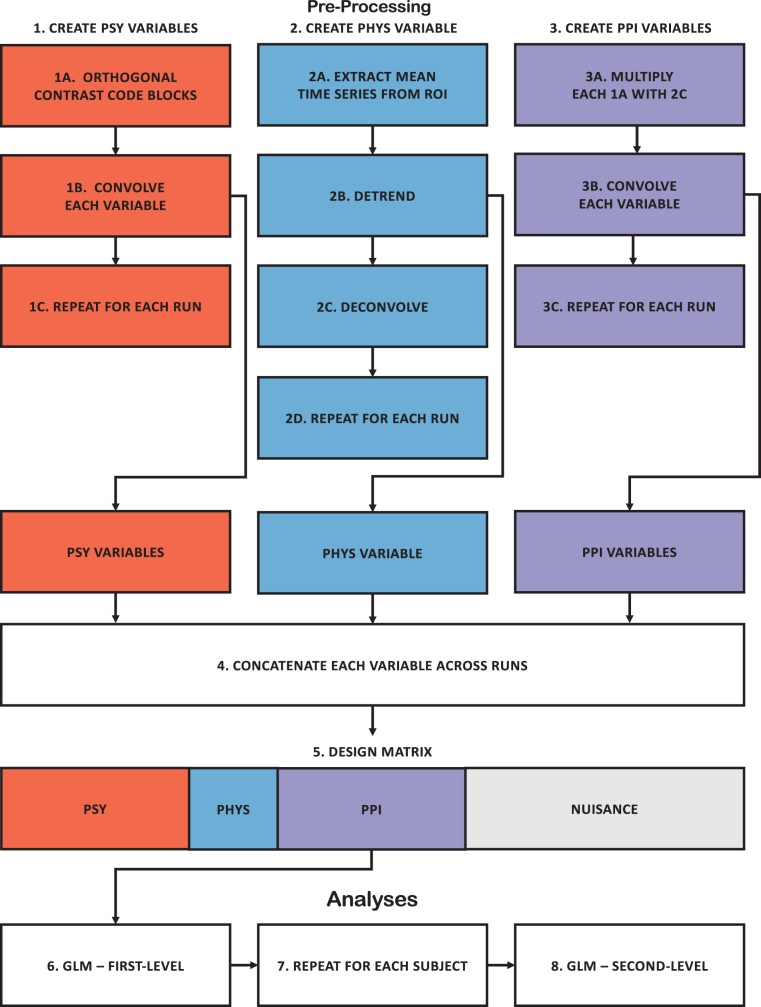
PPI analysis pipeline. To create the PSY variable (Step 1), the time-series of the task was orthogonally contrast coded (1A), each contrast code was then convolved (1B), and these steps were repeated for each run (1C). To create the PHYS variable (Step 2), the mean time-series was extracted from an ROI (2A), detrended linearly and quadratically (2B), deconvolved (2C), and these steps were repeated for each run (2C). To create the PPI variable (Step 3), each contrast code (1A) and deconvolved time-series (2C) was multiplied (3A), convolved (3B), and these steps were repeated for each run (3C). The PSY (1B), PHYS (2B), and PPI variable (3B) were concatenated across runs (4) and used as predictors along with nuisance variables (5) in the first-level GLM analysis (6). Nuisance variables in the GLM analysis included temporal drift, 24-motion parameters, and CSF and white matter time-series along with a high-pass filter. These steps were then repeated for each subject (7). The estimates from the first-level analyses were then used in second-level analyses (8). This pipeline was repeated for each ROI. CSF, cerebral spinal fluid; GLM, general linear model; PHYS, physiological; PPI, psychophysiological interaction; PSY, psychological.
FIG. 1.
Sphere regions of interest within the FP (A) and DM (B) regions. Coordinates for the ROIs were chosen by using the statistical map from the parametric modulation effects reported in Kennedy et al. (2017) using peak global and local maxima from regions that positively modulated within FP regions (A) and negatively modulated within DM regions (B) in response to WM. DM, default-mode; FP, frontal-parietal; ROI, region of interest; WM, working memory.
To confirm that the selected seeds fell within the respective FPN or DMN, a mask was created combining atlases from Neurosynth (Yarkoni et al., 2011), Yeo's 7 Networks (Yeo et al., 2011), CAREN (Doucet et al., 2019), and Shirer's 90 functional ROIs (Shirer et al., 2012). For the Neurosynth atlas, the FPN mask combined a mask of the phrases: “working memory,” “fronto parietal,” “frontoparietal,” and “frontoparietal network,” whereas the DMN mask combined a mask of the phrases: “default,” “default mode,” “default network,” “dmn,” and “network dmn.” All selected seeds fell within their respective networks, with the exception of ROI 15 (supramarginal gyrus) that fell outside the DMN mask; however, the pattern of results was identical with the inclusion or exclusion of this ROI. Although these ROIs were extracted from the parametric modulation to difficulty contrast, most of these regions were also found to be age-sensitive (exceptions included ROIs 9–12 within the FP ROIs).
PPI analyses
To examine FC, modified gPPI methods (Cisler et al., 2014; Di et al., 2017, 2018; Friston et al., 1997; McLaren et al., 2012) were implemented in Analysis of Functional NeuroImages software (Chen, 2015; Cox, 1996) utilizing a priori orthogonal contrasts (Kaufman and Sweet, 1974; Lewis and Mouw, 1972). Second-level analyses were performed by using the stats package and visualized by using the ggplot2 package in R via RStudio (R Core Team, 2020; RStudio Team, 2018; Wickham, 2016).
We utilized gPPI methods to examine whether the blood-oxygen-level-dependent (BOLD) activity between two ROIs has a strengthened correlation (strengthened positive or negative correlation of activity across time) during a given task contrast using the pipeline illustrated in Figure 2. To create the psychological condition regressor, n-back working memory load (0-, 2-, 3-, 4-back), along with fixation, was treated as a categorical variable and a priori orthogonal contrasts codes were created. Specifically, contrasts were specified to test the effects of (1) n-back (0-, 2-, 3-, 4-back) compared with fixation (fixation: −0.800; 0-back: 0.200; 2-back: 0.200; 3-back: 0.200; 4-back: 0.200), (2) the task (2-, 3-, and 4-back) compared with control (0-back) condition (fixation: 0.000; 0-back: −0.750; 2-back: 0.250; 3-back: 0.250; 4-back: 0.250), (3) the linear parametric (slope) effect of task (fixation: 0.000; 0-back: 0.000; 2-back: −0.500; 3-back: 0.000; 4-back: 0.500), and (4) the quadratic parametric (slope) effect of task (fixation: 0.000; 0-back: 0.000; 2-back: −0.333; 3-back: 0.667; 4-back: −0.333). Each psychological contrast was convolved by using the canonical hemodynamic response function (HRF) from SPM. The convolved orthogonal contrast was used as the psychological variable (PSY). The mean BOLD signal was extracted from the seed ROI; the temporal trend (i.e., constant, linear, and quadratic) was removed from the time series, and then deconvolved by using the same canonical HRF. The temporally detrended time series of the seed ROI was used as the physiological regressor (PHYS). To create each PPI regressor, the deconvolved physiological time series and each unconvolved orthogonal contrast were multiplied. The PPI term was then convolved by using the same canonical HRF and used as the PPI variable (PPI). The PSY, PHYS, and PPI regressors were then concatenated across the functional runs. Each voxel's concatenated BOLD time series was then regressed on the PSY, PHYS, and PPI variables while simultaneously controlling for temporal drift (i.e., baseline, linear, and quadratic), 24-motion parameters (Friston et al., 1996), the mean time series extracted from subject specific white matter and cerebral spinal fluid masks, along with a 210s high-pass filter within a general linear model (GLM) framework (Eq. 1). The mean regression coefficient (i.e., unstandardized slope) for each PPI variable was extracted for each seed-to-target ROI (4 regression coefficients [PPI] × 20 seed ROIs × 19 target ROIs = 1,520 regression coefficients per subject).
| (1) |
where the BOLDvoxel dependent variable (DV) represents a subject's voxel's BOLD time series, the four PSY independent variables (IVs) represent each of the convolved a priori orthogonal contrasts, the PHYS IV represents the extracted mean seed ROI time series, the four PPI IVs represent the convolved interaction between the PSY and PHYS variable, and the [Nuisance] IV represents all of the nuisance variables. Each nuisance variable has its own term in the equation, and it is written here collectively as [Nuisance] for simplicity.
Group analyses
To test FC within- and between the FP and DM regions, a group-level analysis was performed on each mean PPI regression coefficient of each seed-to-target ROI combination within a GLM framework (Eq. 2) with the exclusion of when the seed and target ROIs were identical. In other words, the 1,520 estimates were analyzed in an intercept-only model. Given the large number of non-independent analyses, the α-level (Type I error) was adjusted by using the Meff correction (Derringer, 2018). The Meff value of all the PPI variables was calculated to be 1501.79. The Meff value was then used to divide by the overall α of 0.05 to obtain the corrected α of 0.00003.
| (2) |
where the PPIcontrast DV represents the regression coefficient associated with one of the four a priori orthogonal contrasts for each subject from Eq. (1).
For subsequent analyses, PPI regression coefficients of each seed-to-target ROI were averaged to create FC within FP “network” (FPN), FC within DM “network” (DMN), and FC between FPN and DMN for each PPI variable (4 regression coefficients [PPI] × 3 FC pairs = 7 estimates per subject). The Meff correction for these analyses equaled 11.84. Using an overall α of 0.05, the corrected α used was 0.004. To test whether FC within and between the FPN and DMN varied across the adult lifespan, each PPI variable and FC pair were regressed on linear and quadratic age (Eq. 3). Linear age was mean-centered, and quadratic age was the square of mean-centered age. To test whether there was an association between FC and n-back task performance across the adult lifespan, n-back performance using d′ was regressed on FC of each PPI variable and network pair, age, and quadratic age along with its interactions (Eq. 4). FC of each PPI variable and network pair was mean-centered across participants. d′ was calculated as z(hit rate)−z(false alarm rate). False alarm rates of 0 were adjusted by using 1/(2N), where N was the total number of possible false alarms (i.e., N = 276), whereas hit rates of 1 were adjusted by using 1 − 1/(2N) where N represented the total number of possible hits (i.e., N = 144). Lastly, to determine whether the relationship between FC and WM performance was generalizable to out-of-scanner WM performance, DS sequencing performance was regressed on FC of each PPI variable and network pair, age, and quadratic age along with its interactions (Eq. 5).
| (3) |
where the PPIcontrast DV is the same DV as described in Eq. (2), the Age IV represents the mean-centered age, and the Age2 IV represents the square of the mean-centered Age
| (4) |
where the d′ DV is the discriminability index of the in-scanner n-back task. The PPIcontrast, Age, and Age2 variables are the same variables as described in Eq. (3).
| (5) |
where the DS sequencing DV is the out-of-scanner task accuracy. The PPIcontrast, Age, and Age2 variables are the same variables as described in Eq. (3).
Results
FC during n-back
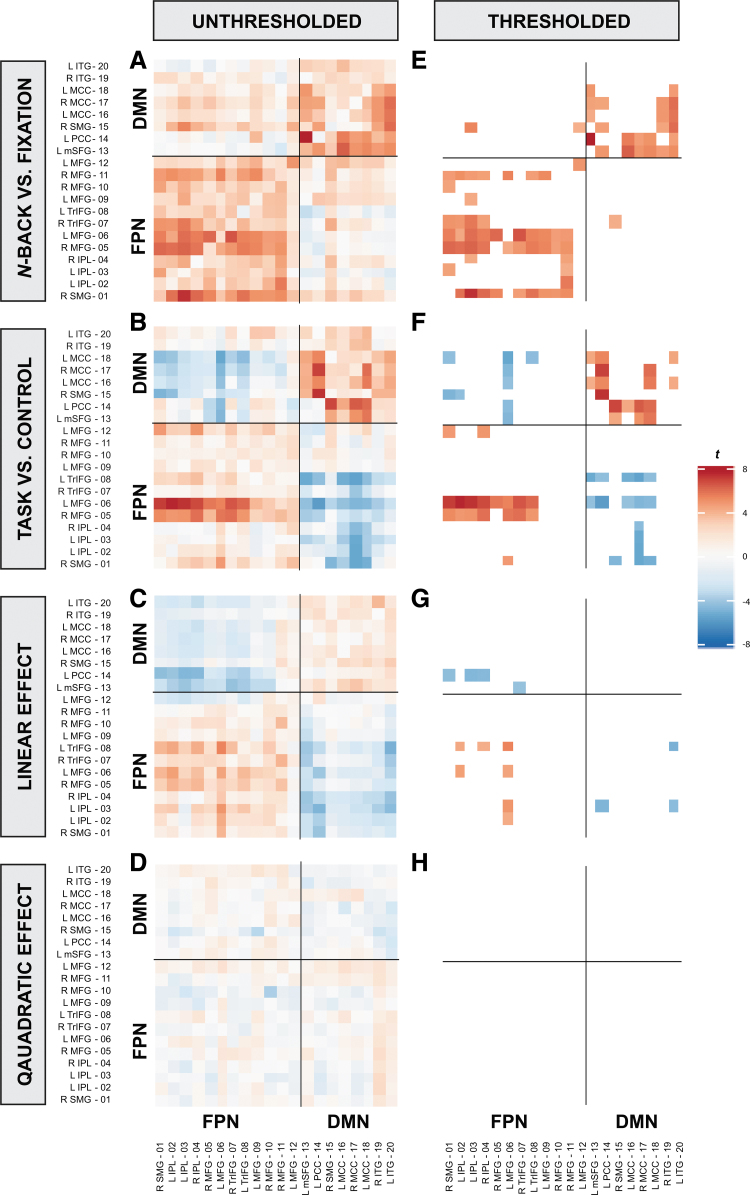
To test for significant FC within- and between FP and DM regions during n-back, the regression coefficient of each PPI variable and seed-to-target ROI combination was analyzed in an intercept-only model. Figure 3 displays both the unthresholded and thresholded results. Significant, thresholded results are summarized next as a range between the minimum regression coefficient (b) and its respective statistics (i.e., t-statistic and adjusted partial r2) to the maximum b and its respective statistics for each PPI variable and FC region.
FIG. 3.
Unthresholded (A–D) and thresholded (E–H) t-statistics of each ROI pair for each PPI variable. Across PPI variables, a generally negative correlation pattern is observed between FPN and DMN whereas a positive correlation pattern is displayed within-FPN and within-DMN (with the exception of the n-back vs. fixation contrast). These effects were significant for the n-back versus fixation (E), the task versus control (F), and the linear effect of task contrasts (G). However, the positive correlation within-DM was not significant for the linear effect of task contrast (G, top-right quadrant). Each row represents each PPI variable, whereas the left column (A–D) represents unthresholded values and the right column (E–H) represents the thresholded values. For each heatmap, the x-axis represents the seed ROIs and the y-axis represents the target ROIs, which are clustered into FPN and DMN groups. For each heatmap, the bottom-left quadrant represents FC within-FPN, the top-right quadrant represents FC within-DMN, whereas the remaining top-left and bottom-right represents FC between-FPN-DMN. Significant effects were thresholded by using an αMeff of 0.00003. DMN, default-mode network; FPN, fronto-parietal network; IPL, inferior parietal lobule; ITG, inferior temporal gyrus ; L, left; MCC, middle cingulate cortex; MFG, middle frontal gyrus; mSFG, medial superior frontal gyrus; PCC, posterior cingulate cortex; R, right; SMG, supramarginal gyrus; TrIFG, inferior frontal triangularis.
Regions within the FPN and within the DMN, respectively, showed significant positive FC (positive correlation; in-phase synchronization) during the n-back compared with fixation, [significant FC within-FPN: b = 0.20, t(169) = 5.18, adjusted rp2 = 0.14 to b = 1.55, t(169) = 5.68, adjusted rp2 = 0.16; significant FC within-DMN: b = 0.41, t(169) = 5.11, adjusted rp2 = 0.13 to b = 1.67, t(169) = 6.67, adjusted rp2 = 0.21], as well as during task compared with the control condition [significant FC within-FPN: b = 0.68, t(169) = 5.67, adjusted rp2 = 0.16 to b = 1.32, t(169) = 6.01, adjusted rp2 = 0.18; significant FC within-DMN: b = 0.48, t(169) = 4.57, adjusted rp2 = 0.11 to b = 2.86, t(169) = 7.24, adjusted rp2 = 0.24]. Regions within FPN revealed significant strengthened positive FC as n-back load increased, but not within DMN [significant FC within-FP: b = 0.31, t(169) = 4.69, adjusted rp2 = 0.12 to b = 0.59, t(169) = 5.14, adjusted rp2 = 0.14]. There were no significant quadratic task effects of FC within-FP or within-DM regions.
Regions between FPN and DMN revealed significant strengthened negative FC (negative correlation; anti-phase synchronization) during the n-back compared with fixation [significant: b = 0.57, t(169) = 4.28, adjusted rp2 = 0.10 to b = 0.98, t(169) = 4.36, adjusted rp2 = 0.10], during the task compared with the control condition [significant: b = −1.84, t(169) = −4.85, adjusted rp2 = 0.12 to b = −0.55, t(169) = −4.40, adjusted rp2 = 0.10], and during the linear effect of task [significant: b = −0.69, t(169) = −4.51, adjusted rp2 = 0.11 to b = −0.37, t(169) = −4.58, adjusted rp2 = 0.11]. FC between FP and DM regions was not significant in the quadratic effect of task. Thus, regions within the FPN and DMN, along with regions between FPN and DMN were functionally connected to a greater extent during the task as compared with fixation or control, and additionally as a function of increasing n-back load.
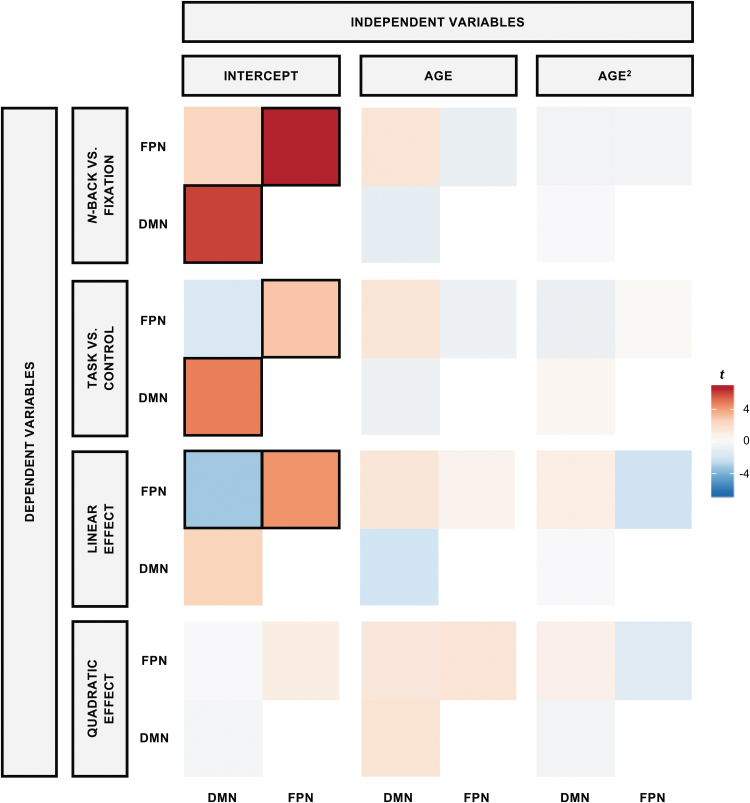
FC during n-back by age
To determine whether FC within- and between FPN and DMN during the n-back was moderated by age, each PPI variable and network pair (i.e., mean regression coefficient within-FPN, within-DMN, and between FPN and DMN) were regressed on linear and quadratic age (Fig. 4 and Table 3). Using a corrected α of 0.004, FC within- and between FPN and DMN did not significantly change with linear (p's > 0.022) or quadratic age (p's > 0.020), suggesting that both within- and between FP and DM connectivity during tasks were age-invariant. See Supplementary Figures S1 and S2 for the results applying a liberal, uncorrected α threshold of 0.05.
FIG. 4.
Effects of age on FC. Across all four tested models, there were no age differences in FC within- or between- FPN or DMN, indicating robustness of connectivity across the adult lifespan. t-statistics for each PPI variable for each FC network pair (DV) for linear and quadratic age are illustrated. Each row represents each DV (i.e., PPI variable), whereas each column represents each IV. For each heatmap, the t-statistic of the mean FC within-DMN is represented in the bottom-left quadrant, the mean FC within-FPN is represented in the top-right quadrant, and the mean FC between FPN-DMN is represented in the top-left quadrant. Significant estimates, which were thresholded by using an αMeff of 0.004, are indicated with black boxes. DV, dependent variable; FC, functional connectivity; IV, independent variable.
Table 3.
Second-Level Analysis of Each Psychophysiological Interaction Variable and Functional Connectivity by Linear and Quadratic Age
| PPI variable | FC | Variable | b | SE | t(167) | p |
|---|---|---|---|---|---|---|
| Back vs. fixation | Within FPN | Intercept | 0.61 | 0.09 | 6.90 | <0.001* |
| Age | 0.00 | 0.00 | −1.05 | 0.295 | ||
| Age2 | 0.00 | 0.00 | −0.56 | 0.573 | ||
| Within DMN | Intercept | 0.61 | 0.10 | 6.22 | <0.001* | |
| Age | 0.00 | 0.00 | −1.21 | 0.228 | ||
| Age2 | 0.00 | 0.00 | −0.13 | 0.896 | ||
| Between FPN and DMN | Intercept | 0.26 | 0.11 | 2.50 | 0.013 | |
| Age | 0.01 | 0.00 | 1.63 | 0.105 | ||
| Age2 | 0.00 | 0.00 | −0.62 | 0.535 | ||
| Task vs. control | Within FPN | Intercept | 0.32 | 0.11 | 3.05 | 0.002* |
| Age | 0.00 | 0.00 | −0.83 | 0.407 | ||
| Age2 | 0.00 | 0.00 | 0.27 | 0.786 | ||
| Within DMN | Intercept | 0.58 | 0.12 | 4.87 | <0.001* | |
| Age | 0.00 | 0.00 | −0.87 | 0.385 | ||
| Age2 | 0.00 | 0.00 | 0.51 | 0.613 | ||
| Between FPN and DMN | Intercept | −0.21 | 0.11 | −1.82 | 0.071 | |
| Age | 0.01 | 0.00 | 1.55 | 0.122 | ||
| Age2 | 0.00 | 0.00 | −0.98 | 0.330 | ||
| Linear task | Within FPN | Intercept | 0.21 | 0.05 | 4.41 | <0.001* |
| Age | 0.00 | 0.00 | 0.65 | 0.514 | ||
| Age2 | 0.00 | 0.00 | −2.36 | 0.020 | ||
| Within DMN | Intercept | 0.14 | 0.05 | 2.56 | 0.012 | |
| Age | 0.00 | 0.00 | −2.30 | 0.022 | ||
| Age2 | 0.00 | 0.00 | −0.08 | 0.936 | ||
| Between FPN and DMN | Intercept | −0.20 | 0.06 | −3.39 | 0.087* | |
| Age | 0.00 | 0.00 | 1.41 | 0.160 | ||
| Age2 | 0.00 | 0.00 | 1.06 | 0.290 | ||
| Quadratic task | Within FPN | Intercept | 0.08 | 0.08 | 1.09 | 0.276 |
| Age | 0.00 | 0.00 | 1.64 | 0.103 | ||
| Age2 | 0.00 | 0.00 | −1.53 | 0.127 | ||
| Within DMN | Intercept | −0.04 | 0.08 | −0.42 | 0.674 | |
| Age | 0.01 | 0.00 | 1.75 | 0.082 | ||
| Age2 | 0.00 | 0.00 | −0.58 | 0.566 | ||
| Between FPN and DMN | Intercept | −0.01 | 0.09 | −0.12 | 0.904 | |
| Age | 0.00 | 0.00 | 1.39 | 0.165 | ||
| Age2 | 0.00 | 0.00 | 0.87 | 0.387 |
Significant effects; p < Meff-corrected α of 0.004.
DMN, default-mode network; FC, functional connectivity; FPN, fronto-parietal network; SE, standard error.
Effects of FC and aging on n-back task performance
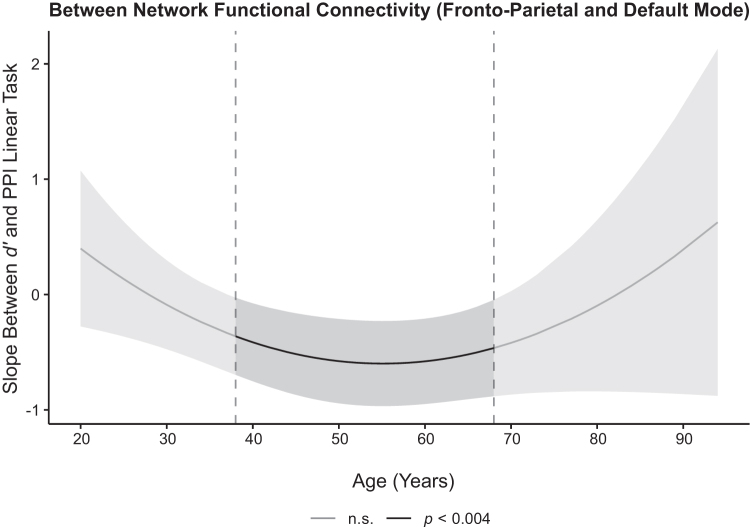
To examine whether FC within- and between the FPN and DMN predicted n-back performance across the lifespan, d′ was regressed on each PPI variable, age, quadratic age, and its interactions for each FC region. Of these analyses, only FC between the FPN and DMN during the linear effect of task significantly predicted d′ (b = −0.60, t(164) = −4.69, p < 0.001, adjusted rp2 = 0.09). Specifically, d′ increased as FC between FPN-DMN became more negatively coupled (increased anti-phase synchronization; FPN increased as DMN decreased). Further, this association was moderated by quadratic age, b = 0.0008, t(164) = 2.99, p = 0.003, adjusted rp2 = 0.02. Post hoc simple slopes analyses of this interaction, which were conducted using the Johnson-Neyman procedure (Preacher et al., 2006) with a confidence interval using an αMeff of 0.004, revealed that the association between d′ and FC between FPN and DMN during the linear effect of task was significant for individuals between the ages of 38 and 68 (p's < 0.0002, adjusted rp2 = 0.03–0.09), suggesting that for middle-aged and older adults FC is a significant factor for performance, whereas it is not a significant predictor of task performance for the younger and oldest adult individuals (Fig. 5 and Table 4). Neither within-FPN nor within-DMN connectivity was associated with d′.
FIG. 5.
Effects of FC on cognitive performance across the adult lifespan. WM d′ during the in-scanner task was significantly predicted by strength of FC between FPN-DMN, but it was age-dependent. Post hoc analyses using the Johnson-Neyman procedure to decompose this significant interaction indicated stronger negative coupling between these regions (i.e., increased FPN and decreased DMN) during increasing WM load and significantly predicted higher d′ scores for middle-aged and older adults (i.e., participants between the ages of 38 and 68). The confidence band represents the confidence interval by using an αMeff of 0.004. d′, discriminability index.
Table 4.
Linear and Quadratic Age, PPI, and Interactions for Each PPI Variable and FC Effects on Working Memory Discrimination Index (d′)
| PPI variable | FC | Variable | b | SE | t(164) | p |
|---|---|---|---|---|---|---|
| Back vs. fixation | Within FPN | Intercept | 1.77 | 0.09 | 18.94 | <0.001* |
| PPI | −0.11 | 0.10 | −1.03 | 0.302 | ||
| Age | −0.02 | 0.00 | −6.00 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.24 | 0.810 | ||
| PPI × age | 0.00 | 0.00 | −0.23 | 0.818 | ||
| PPI × age2 | 0.00 | 0.00 | 0.67 | 0.501 | ||
| Within DMN | Intercept | 1.70 | 0.10 | 17.64 | <0.001* | |
| PPI | 0.02 | 0.09 | 0.25 | 0.803 | ||
| Age | −0.02 | 0.00 | −6.15 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.87 | 0.388 | ||
| PPI × age | 0.00 | 0.00 | −0.30 | 0.765 | ||
| PPI × age2 | 0.00 | 0.00 | −0.51 | 0.612 | ||
| Between FPN and DMN | Intercept | 1.67 | 0.08 | 21.41 | <0.001* | |
| PPI | 0.15 | 0.08 | 1.92 | 0.057 | ||
| Age | −0.02 | 0.00 | −7.54 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 1.13 | 0.259 | ||
| PPI × age | 0.00 | 0.00 | 0.60 | 0.548 | ||
| PPI × age2 | 0.00 | 0.00 | −1.66 | 0.099 | ||
| Task vs. control | Within FPN | Intercept | 1.66 | 0.08 | 20.80 | <0.001* |
| PPI | 0.16 | 0.08 | 2.05 | 0.0422 | ||
| Age | −0.02 | 0.00 | −6.99 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 1.12 | 0.263 | ||
| PPI × age | 0.00 | 0.00 | −0.11 | 0.912 | ||
| PPI × age2 | 0.00 | 0.00 | −1.26 | 0.210 | ||
| Within DMN | Intercept | 1.73 | 0.09 | 18.99 | <0.001* | |
| PPI | −0.03 | 0.07 | −0.37 | 0.711 | ||
| Age | −0.02 | 0.00 | −6.52 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.43 | 0.665 | ||
| PPI × age | 0.00 | 0.00 | −0.14 | 0.893 | ||
| PPI × age2 | 0.00 | 0.00 | 0.42 | 0.677 | ||
| Between FPN and DMN | Intercept | 1.73 | 0.08 | 22.33 | <0.001* | |
| PPI | 0.08 | 0.07 | 1.14 | 0.254 | ||
| Age | −0.02 | 0.00 | −6.49 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.43 | 0.670 | ||
| PPI × age | 0.00 | 0.00 | 0.64 | 0.522 | ||
| PPI × age2 | 0.00 | 0.00 | −0.67 | 0.501 | ||
| Linear task | Within FPN | Intercept | 1.71 | 0.08 | 21.07 | <0.001* |
| PPI | 0.05 | 0.18 | 0.30 | 0.765 | ||
| Age | −0.02 | 0.00 | −7.24 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.78 | 0.434 | ||
| PPI × age | −0.01 | 0.01 | −1.15 | 0.253 | ||
| PPI × age2 | 0.00 | 0.00 | −0.40 | 0.691 | ||
| Within DMN | Intercept | 1.76 | 0.08 | 22.50 | <0.001* | |
| PPI | −0.31 | 0.15 | −2.05 | 0.042 | ||
| Age | −0.02 | 0.00 | −7.25 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.04 | 0.972 | ||
| PPI × age | 0.01 | 0.01 | 0.83 | 0.407 | ||
| PPI × age2 | 0.00 | 0.00 | 2.70 | 0.008 | ||
| Between FPN and DMN | Intercept | 1.62 | 0.08 | 21.37 | <0.001* | |
| PPI | −0.60 | 0.13 | −4.69 | <0.001* | ||
| Age | −0.02 | 0.00 | −8.10 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 1.52 | 0.130 | ||
| PPI × age | 0.00 | 0.01 | −0.61 | 0.544 | ||
| PPI × age2 | 0.00 | 0.00 | 2.99 | 0.003* | ||
| Quadratic task | Within FPN | Intercept | 1.70 | 0.08 | 22.07 | <0.001* |
| PPI | 0.11 | 0.11 | 0.95 | 0.342 | ||
| Age | −0.02 | 0.00 | −7.07 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.73 | 0.464 | ||
| PPI × age | 0.00 | 0.00 | 0.30 | 0.768 | ||
| PPI × age2 | 0.00 | 0.00 | −0.62 | 0.535 | ||
| Within DMN | Intercept | 1.71 | 0.08 | 22.65 | <0.001* | |
| PPI | 0.17 | 0.11 | 1.50 | 0.136 | ||
| Age | −0.02 | 0.00 | −7.70 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.84 | 0.402 | ||
| PPI × age | 0.00 | 0.00 | 0.72 | 0.472 | ||
| PPI × age2 | 0.00 | 0.00 | −0.01 | 0.995 | ||
| Between FPN and DMN | Intercept | 1.72 | 0.08 | 22.15 | <0.001* | |
| PPI | −0.03 | 0.10 | −0.35 | 0.728 | ||
| Age | −0.02 | 0.00 | −7.36 | <0.001* | ||
| Age2 | 0.00 | 0.00 | 0.58 | 0.565 | ||
| PPI × age | 0.00 | 0.00 | −0.27 | 0.787 | ||
| PPI × age2 | 0.00 | 0.00 | 0.65 | 0.518 |
Significant effects; p < Meff-corrected α of 0.04.
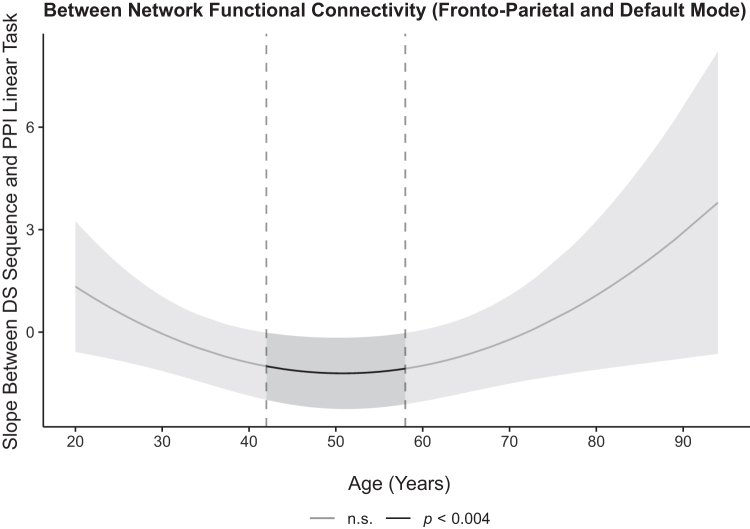
To examine whether FC within- and between FPN and DMN predicted out-of-scanner WM performance across the lifespan, a similar analysis was performed with DS sequencing scores as the dependent variable. Similar results were found with only the linear effect of task significantly predicting DS sequencing performance [b = −1.12, t(164) = −3.32, p = 0.001, adjusted rp2 = 0.03]. Specifically, DS sequencing performance increased as FC between FPN and DMN became more negatively coupled (strengthened anti-phase synchronization). Further, this association was moderated by quadratic age, b = 0.0027, t(164) = 3.405, p < 0.001, adjusted rp2 = 0.04. Using post hoc analyses as previously described revealed that the association between DS sequencing performance and FC between FPN and DMN during the linear effect of task was significant in individuals between the ages of 42 and 58 years (p's < 0.0035, adjusted rp2 = 0.02–0.04), suggesting the importance of FC for middle-aged and older adults' WM performance, but not for the younger and oldest individuals (Fig. 6). Within-FPN and within-DMN connectivity was not associated with sequencing performance. Taken together, FC between FPN and DMN was associated with both in- and out-of-scanner WM performance, specifically for middle and older adults, but not younger and oldest adults.
FIG. 6.
Effects of FC on out-of-scanner WM performance across the adult lifespan. DS sequencing performance measured outside the scanner was significantly predicted by strength of FC between FPN and DMN, but it was age-dependent. Post hoc using the Johnson-Neyman procedure analyses revealed that stronger negative coupling between FPN and DMN was associated with better WM performance in individuals aged 42–58. The confidence band represents the confidence interval by using an αMeff of 0.004. DS, digit span.
Discussion
Here, we report that connectivity within FP regions becomes more in-phase synchronous both when engaging in working memory on average and as WM load increases, and that connectivity within the DM regions becomes more in-phase synchronous when engaging in WM. Further, connectivity between the FPN and DMN became more anti-phase synchronous (i.e., more negatively coupled) both when engaging in WM and as load increased. Strengthening of connectivity within-FPN during WM and in response to WM load aligns with previous studies (Heinzel et al., 2014, 2017; Nagel et al., 2011; Sala-Llonch et al., 2012) that examined groups of younger versus older adults. The current findings are also in accord with a meta-analysis of PPI studies across cognitive control tasks, including working memory, compared with other cognitive domains that indicated increased FC within FP regions (i.e., dorsolateral prefrontal cortex and posterior cingulate cortex) (Smith et al., 2016). Together, these results support the general notion that regions within the FPN and DMN become functionally integrated during task engagement (Shine et al., 2016).
Interestingly, FC within- and between FPN and DMN were robust to the effects of cross-sectional aging across the adult lifespan in the current investigation. The extant literature of age effects on FC during the n-back task shows inconsistent associations. Older adults have been reported to show decreased (Heinzel et al., 2014, 2017; Nagel et al., 2011) and increased FC within FPN (Heinzel et al., 2014, 2017) compared with younger adults. In addition, older adults have also been reported to show weakened and reversed FC between FPN and DMN (Hakun et al., 2015; Turner and Spreng, 2015) compared with younger adults. The addition of the current adult lifespan study to the inconsistent extreme age group findings in the literature suggests that there may not be true cross-sectional age effects on FC within-FPN.
These working memory findings parallel work in the episodic memory domain where age effects on FC are also inconsistently reported, with some studies observing decreases in FC with aging using age group comparisons (Foster et al., 2016; St. Jacques et al., 2012; King et al., 2018; Tsukiura et al., 2011), others finding increases in aging using age group comparisons (Foster et al., 2016; King et al., 2018; Oh and Jagust, 2013; Trelle et al., 2019), and some reporting age invariance in FC (Trelle et al., 2019). Thus, for the episodic memory literature, as also demonstrated in working memory, FC differences in task sensitive regions are mixed or lack clear directionality. The lack of consistent findings in multiple literatures, as well as the absence of age effects in the current study that utilized a large adult lifespan sample, provides evidence that FC may be an age-invariant brain property.
We speculate there are several ostensible reasons for the present age-invariance compared with the mixed literature. First, there are likely additional null cross-sectional age findings during n-back that have not been published given the “file-drawer problem” (Rosenthal, 1979) that has persisted into a “replication crisis” (Lindsay, 2015). However, further replication, particularly in longitudinal samples that are more sensitive and more precisely capture individual aging effects, are warranted (and is currently underway for the current sample). Second, our findings of age invariance in FC may also be due to differences in sample characteristics in the current sample versus some of those in the literature. For example, in addition to the inclusion and exclusion criteria of the aforementioned studies (Heinzel et al., 2014, 2017; Nagel et al., 2011), the current study additionally explicitly excludes participants for cardiovascular disease (except for controlled essential hypertension), head trauma with loss of consciousness, and diabetes. These health conditions alone have been shown to alter task-free FC compared with healthy controls (Li et al., 2015; Yang et al., 2016; Zhang et al., 2016). Although head trauma is a common study exclusion and may not be explicitly stated in all articles, prior studies mention the inclusion of uncontrolled and controlled cardiovascular disease and diabetes in their sample (Nagel et al., 2011). Thus, we speculate that the lack of an age effect on FC may be due to our sample representing the healthy end of the normal aging continuum, whereas other samples may represent typical aging, including the influence of some common age-related comorbidities (Meusel et al., 2014). Additional differences in our sample include the study of middle-aged adults, who were typically excluded in prior studies. Third, the differences in age effects reported in the literature may stem from diverse methodological approaches and thresholding stringencies applied across studies. For example, the current study implemented a fairly stringent correction for multiple comparisons to mitigate Type I error, whereas most of the published aging articles utilized no alpha correction (with the exception of Heinzel et al., 2017), leaving the possibility that some effects may be spurious and influenced by Type I error. Multiple methodologies have been applied to the computation of FC, including standard PPI, gPPI, granger causality, and so forth, introducing additional method variance across the literature. There are also differences in localized ROI selection such as lateralized frontal regions versus a large bilateral working memory network. However, the ROIs of the current study generalize to the FPN and DMN given their inclusion in several masks. A well-controlled meta-analysis could help tease these effects apart.
Intriguingly, although FC may be robust to the effects of age, both positive and negative parametric BOLD modulation during n-back are adversely affected by aging (Kennedy et al., 2017). It is interesting to speculate what the differences in these BOLD metrics might represent. BOLD activation, and modulation of activation to difficulty reflect a magnitude-level effect of changes in oxy-deoxyhemoglobin from one state to the next, as a proxy for neurovascular coupling at the neuronal unit. In contrast, FC is a proxy for functional integration of different neuronal populations in a time-linked fashion (Friston, 2011). It is plausible that biological mechanisms induce age-related constraints or limitations on increasing magnitude of BOLD activation, whereas different mechanisms underlie functional integration, such as white matter connections across the cortex and subcortical regions. Although these white matter connections are degraded with even healthy aging (Bennett and Madden, 2014; Kennedy and Raz, 2015), frank loss of axons or neuronal cell bodies is not observed in healthy aging (Liu et al., 2017). Functional integration may be maintained in this healthy sample, because white matter tracts still provide an avenue for long-range functional integration.
In our prior report, a significant coupled association between positive modulation in FP regions and negative modulation in DM regions did not vary by age in this same sample of participants (Kennedy et al., 2017; Rieck et al., 2017). Thus, in separate investigations, BOLD modulation to cognitive difficulty is significantly altered with aging; however, both coupling of modulation and FC between FP and DM is age-invariant. However, changes in functional integration might be seen with further diminished white matter integrity, outside of healthy aging, as in individuals with mild cognitive impairment or Alzheimer's disease (Wang et al., 2015; Wee et al., 2012).
In addition to characterizing how task-related connectivity behaves across the lifespan, it is also crucial to yoke this connectivity to performance (Grady, 2012). Here, we report that FC within FPN or DMN did not predict WM performance, rather the strengthening of FC between FPN and DMN as task load increased was significantly associated with better in- and out-of-scanner task performance. Middle-aged and older adults who further increased activity in FPN and simultaneously decreased activity in DMN to increasing WM load achieved higher accuracy during n-back performance and DS sequencing. Regardless of FC between FP and DM regions, the youngest adults appeared to perform well, whereas the oldest adults performed poorer. Potentially, anti-phase synchronization (i.e., coupling) between FPN and DMN regions may not be as important to n-back performance in the youngest or the oldest adults compared with adults in the middle to older age range, a period theorized to be crucial for brain maintenance and/or compensation mechanisms (Cabeza et al., 2018).
This pattern of performance associated with FC between FPN and DMN regions is partially aligned with the DECHA model, which states that FPN activation coupled with DMN suppression during executive function tasks is associated with better performance (Turner and Spreng, 2015). We have previously shown in this sample that greater coupling of positive and negative BOLD modulation to difficulty was associated with higher fluid intelligence (Rieck et al., 2017). Our findings also partially align with the finding that increased global functional integration during difficult external tasks such as the n-back paradigm is associated with effective performance (Shine et al., 2016). In addition, previous findings (Kennedy et al., 2017) suggest that strengthened activation of FPN to increasing n-back load (positive modulation) is associated with better performance for middle-aged to oldest adults, but not younger adults, who performed generally well regardless of strengthened activation of FP to increasing n-back load; whereas suppression (deactivation) of DMN to increasing n-back load (negative modulation) is associated with better performance regardless of age. Given the unique information provided by BOLD modulation and FC analyses in respect to both aging and performance, the continued study of both is warranted, especially within longitudinal individual difference studies.
Conclusion
In sum, the current study revealed that FC within FP and DM regions was strengthened (became more positively correlated or in-phase synchronous) when engaging in working memory compared with control, and that FC within FP regions was further strengthened as working memory load increased. In addition, negative FC between the FP and DM was strengthened (became more anti-phase synchronous) when engaging in the WM task compared with control and as WM load increased. Notably, these patterns of connectivity were age-invariant across the adult lifespan. Importantly, however, the association of connectivity strength was linked to task performance in an age-dependent manner. Specifically, the stronger the negative coupling between FPN and DMN as task load increased, the more accurate was the task performance, selectively in middle- to older-aged adults, that is, portions of the age span that are often excluded from most “aging” samples. Further replication supporting the maintenance of functional integration across healthy cognitive and brain aging is warranted, particularly using longitudinal studies to quantify within-person change.
Supplementary Material
Authors' Contributions
E.E.P., K.M.K., and K.M.R. conceived and outlined the idea. E.E.P. processed, analyzed, and visualized the data and results. All authors provided critical feedback and assisted in refining the study, analysis, and article.
Acknowledgments
The authors would like to thank Andy Hebrank for assistance with fMRI task programming, Asha Unni for behavioral task piloting and fMRI data collection, and Marci Horn for cognitive data collection.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported, in part, by grants from the National Institutes of Health: R00 AG-036818, R00 AG-036848, R01 AG-056535, and R01 AG-057537.
Supplementary Material
References
- Artuso C, Cavallini E, Bottiroli S, Palladino P. 2017. Updating working memory: memory load matters with aging. Aging Clin Exp Res 29:371–377 [DOI] [PubMed] [Google Scholar]
- Baddeley A. 2000. The episodic buffer: a new component of working memory? Trends Cogn Sci 4:417–423 [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. 1974. Working memory. Psychol Learn Motiv 8:47–89 [Google Scholar]
- Bennett IJ, Madden DJ. 2014. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience 276:187–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, et al. . 2018. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci 19:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang JH, Dai ZJ, Cao XY, Jiang LL, Fan FM, et al. . 2014. Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci 7:76–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. 2015. AFNI: context-dependent correlation analysis or generalized PPI. http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html Last accessed May01, 2018
- Cisler JM, Bush K, Steele JS. 2014. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. NeuroImage 84:1042–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res Int J 29:162–173 [DOI] [PubMed] [Google Scholar]
- Derringer J. 2018. A simple correction for non-independent tests. 10.31234/osf.io/f2tyw Last accessed April16, 2018 [DOI]
- Di X, Biswal BB. 2018. Toward task connectomics: examining whole-brain task modulated connectivity in different task domains. Cereb Cortex 29:1572–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Reynolds RC, Biswal BB. 2017. Imperfect (de)convolution may introduce spurious psychophysiological interactions and how to avoid it. Human Brain Mapp 38:1723–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Zhang Z, Biswal BB. 2018. Psychophysiological interaction and beta series correlation for task modulated connectivity: modeling considerations and their relationships, bioRxiv:1–29
- Dobbs AR, Rule BG. 1989. Adult age differences in working memory. Psychol Aging 4:500–503 [DOI] [PubMed] [Google Scholar]
- Doucet GE, Lee WH, Frangou S. 2019. Evaluation of the spatial variability in the major resting-state networks across human brain functional atlases. Human Brain Mapp 40:4577–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas J, Nielsen C, Hartman M. 2001. Age differences in updating working memory: evidence from the delayed-matching-to-sample test. Aging Neuropsychol Cogn 8:14–35 [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- Foster CM, Picklesimer ME, Mulligan NW, Giovanello KS. 2016. The effect of age on relational encoding as revealed by hippocampal functional connectivity. Neurobiol Learn Memory 134:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. 2011. Functional and effective connectivity: a review. Brain Connect 1:13–36 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. 1997. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 6:218–229 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. 1996. Movement-Related effects in fMRI time-series. Magn Reson Med 35:346–355 [DOI] [PubMed] [Google Scholar]
- Grady C. 2012. The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakun JG, Zhu Z, Johnson NF, Gold BT. 2015. Evidence for reduced efficiency and successful compensation in older adults during task switching. Cortex 64:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Lorenz RC, Brockhaus W.-R., Wüstenberg T, Kathmann N, Heinz A, Rapp MA.. 2014. Working memory load-dependent brain response predicts behavioral training gains in older adults. J Neurosci 34:1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Lorenz RC, Duong Q-L, Rapp MA, Deserno L. 2017. Prefrontal-parietal effective connectivity during working memory in older adults. Neurobiol Aging 57:18–27 [DOI] [PubMed] [Google Scholar]
- Honey GD, Fu CHY, Kim J, Brammer MJ, Croudace TJ, Suckling J, et al. . 2002. Effects of verbal working memory load on corticocortical connectivity modeled by path analysis of functional magnetic resonance imaging data. NeuroImage 17:573–582 [PubMed] [Google Scholar]
- Kaufman D, Sweet R. 1974. Contrast coding in least squares regression analysis. Am Educ Res J 11:359–377 [Google Scholar]
- Kennedy KM, Boylan MA, Rieck JR, Foster CM, Rodrigue KM. 2017. Dynamic range in BOLD modulation: lifespan aging trajectories and association with performance. Neurobiol Aging 60:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. 2015. Normal Aging of the Brain. In: Toga, AW (ed.) Brain Mapping: An Encyclopedic, vol. 3. Academic Press, NY: Elsevier; p. 603–617 [Google Scholar]
- King DR, de Chastelaine M, Rugg MD. 2018. Recollection-related increases in functional connectivity across the healthy adult lifespan. Neurobiol Aging 62:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EL, Mouw JT. 1972. The use of contrast coding to simply ANOVA and ANCOVA procedures in multiple linear regression. In: Meeting of the American Educational Research Association [Google Scholar]
- Li X, Liang Y, Chen Y, Zhang J, Wei D, Chen K, et al. . 2015. Disrupted frontoparietal network mediates white matter structure dysfunction associated with cognitive decline in hypertension patients. J Neurosci 35:10015–10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS. 2015. Replication in psychological science. Psychol Sci 26:1827–1832 [DOI] [PubMed] [Google Scholar]
- Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. 2017. Aging of cerebral white matter. Ageing Res Rev 34:64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika PK, Whitfield S, Cooper JC. 2005. Detection and repair of transient artifacts in fMRI data. Neuroimage 26:S36 [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage 61:1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusel LAC, Kansal N, Tchistiakova E, Yuen W, MacIntosh BJ, Greenwood CE, Anderson ND. 2014. A systematic review of type 2 diabetes mellitus and hypertension in imaging studies of cognitive aging: time to establish new norms. Front Aging Neurosci 6:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li S-C, Nyberg L, Bäckman L, Lindenberger U, Heekeren HR. 2011. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci 23:2030–2045 [DOI] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC. 2011. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Human Brain Mapp 32:1649–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J. 2016. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. NeuroImage 133:321–330 [DOI] [PubMed] [Google Scholar]
- Oh H, Jagust WJ. 2013. Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. J Neurosci 33:18425–18437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapp 25:46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. 2002. Models of visuospatial and verbal memory across the adult life span. Psychol Aging 17:299–320 [PubMed] [Google Scholar]
- Peirce JW. 2007. PsychoPy—psychophysics software in Python. J Neurosci Methods 162:8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. 2009. Generating stimuli for neuroscience using PsychoPy. Front Neuroinform 2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. 2006. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat 31:437–448 [Google Scholar]
- R Core Team. 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Radloff LS. 1977. A self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- Rieck JR, Rodrigue KM, Boylan MA, Kennedy KM. 2017. Age-related reduction of BOLD modulation to cognitive difficulty predicts poorer task accuracy and poorer fluid reasoning ability. NeuroImage 147:262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. 1979. The file drawer problem and tolerance for null results. Psychol Bull 86:638–641 [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, et al. . 2012. Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage 60:830–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team. 2018. RStudio: Integrated Development for R. R for Beginners. Boston, MA: RStudio Team [Google Scholar]
- Sala-Llonch R, Peña-Gómez C, Arenaza-Urquijo EM, Vidal-Piñeiro D, Bargalló N, Junqué C, Bartrés-Faz D. 2012. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex 48:1187–1196 [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, et al. . 2010. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci 22:655–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, et al. . 2016. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron 92:544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. 2012. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex 22:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Gseir M, Speer ME, Delgado MR. 2016. Toward a cumulative science of functional integration: a meta-analysis of psychophysiological interactions. Human Brain Mapp 37:2904–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Rubin DC, Cabeza R. 2012. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol Aging 33:1298–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson HL. 1999. What develops in working memory? A life span perspective. Dev Psychol 35, 986–1000 [DOI] [PubMed] [Google Scholar]
- Trelle AN, Henson RN, Simons JS. 2019. Neural evidence for age-related differences in representational quality and strategic retrieval processes. Neurobiol Aging 84:50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Sekiguchi A, Yomogida Y, Nakagawa S, Shigemune Y, Kambara T, et al. . 2011. Effects of aging on hippocampal and anterior temporal activations during successful retrieval of memory for face-name associations. J Cogn Neurosci 23:200–213 [DOI] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. 2015. Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: the default–executive coupling hypothesis of aging. J Cogn Neurosci 27:2462–2476 [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. 2003. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 3:255–274 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang J, Zhang H, Mchugh R, Sun X, Li K, Yang QX. 2015. Interhemispheric functional and structural disconnection in Alzheimer s disease: A combined resting-state fMRI and DTI study. PLoS ONE 10:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2008. Wechsler Adult Intelligence Scale, 4th ed. San Antonio, TX: Pearson [Google Scholar]
- Wee CY, Yap PT, Zhang D, Denny K, Browndyke JN, Potter GG, Welsh-Bohmer KA, Wang L, Shen D. 2012. Identification of MCI individuals using structural and functional connectivity networks. NeuroImage 59:2045–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. 2016. ggplot2 Elegant Graphics for Data Analysis. New York: Springer-Verlag New York [Google Scholar]
- Yang SQ, Xu ZP, Xiong Y, Zhan YF, Guo LY, Zhang S, et al. . 2016. Altered intranetwork and internetwork functional connectivity in type 2 diabetes mellitus with and without cognitive impairment. Sci Rep 6:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Essen DC. Van Wager TD. 2011. NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Front Neuroinform 5; DOI: 10.3389/conf.fninf.2011.08.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. . 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu S, Liu C, Zhang H, Zhou X, Ni C, et al. . 2016. Altered brain activation and functional connectivity in working memory related networks in patients with type 2 diabetes: an ICA-based analysis. Sci Rep 6:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.