Thanks to microwave digestion and its ability to quickly and safely heat to high temperatures, the runtime compared to open-vessel techniques is significantly lower. This is because higher temperatures correlate with shorter digestion times. There are various systems available for microwave digestion. These differ in the technology and in the setup of the cavity used, and provide varying levels of sample throughput.
Microwave Digestion
Microwave digestion is a special and successful way of sample preparation for elemental analysis. Whenever samples need to be analyzed for trace elements, they need to be transferred into liquid form in order to be handled by the analytical equipment. This link between sample and analysis is called sample preparation.
The basic technique behind microwave-assisted sample preparation is acid digestion, which is one of the most important sample preparation techniques. By means of acid digestion, a sample is destroyed or dissolved by the use of acids and only the (trace) metals remain in solution. This transfers samples into an analyzable liquid, which is essential because clear, particle-free solutions are required for the analytical equipment to determine small concentrations of toxic metals.
The demands for microwave digestion are high – it needs to be a highly reliable method in order not to compromise analytical data.[1] Due to effective heating with microwaves, reliable reaction control, and high temperature and pressure tolerance, analysis of things like food, soil, and pharmaceuticals is possible, which supports the success of subsequent elemental analysis.
General considerations about microwave digestion
The importance of digestion for successful elemental analysis
There has been a rapid increase in the demand of microwave digestion and elemental analysis over the last few decades. The exponential growth of regulations and norms, an increasing human population, and the global distribution of goods pose enormous challenges for safety, quality, and proving the authenticity of various products. All of this can be investigated with analytical techniques. However, although analytical instruments continue to offer high resolution, fast analysis, robustness, miniaturization, and portability, conducting actual analysis with these instruments still requires extensive sample preparation (i.e., microwave digestion). Obtaining precise results requires appropriate sample preparation because errors made in sampling, sample preparation, or sample introduction (injection) cannot be corrected by even the most advanced analytical system.
Despite many advances in recent years, sample preparation is still often considered to be the bottleneck in the analytical workflow since it takes up more than 60 % of the time needed from sampling to the end result (see Figure 1).
What is acid digestion?
Acid digestion is the most commonly used wet-chemical microwave sample preparation technique. By using concentrated acids or mixtures thereof, the matrices of organic and inorganic samples can be totally destroyed or dissolved, and the whole sample can be brought into solution. Subsequently, the concentration of elements or species can be determined with an adequate analytical technique. For example: AAS (atomic absorption spectroscopy), MIP-OES (microwave-induced plasma optical emission spectrometry), ICP-OES (inductively coupled plasma optical emission spectrometry), ICP-MS (inductively coupled plasma mass spectrometry).[2]
Below is a list of the most commonly used acids for acid digestion and their typical concentrations:[3]
- Nitric acid, HNO3 (65 %)
- Hydrochloric acid, HCl (30 % to 37 %)
- Hydrofluoric acid, HF (40 % to 48 %)
- Sulfuric acid, H2SO4 (95 % to 98 %)
- Perchloric acid, HClO4 (70 % to 72 %)
- Phosphoric acid, H3PO4 (85 %)
- Hydrogen peroxide, H2O2 (30 %)
- Aqua regia, HCl + HNO3 (volume ratio 3:1)
- Reverse aqua regia, HCl + HNO3 (volume ratio 1:3)
- Boric acid, H3BO3 (approx. 5 %)
What is acid leaching?
Acid leaching is – like acid digestion – a commonly used method in microwave-assisted sample preparation. It is a bit similar to acid digestion, but it does not completely destroy or dissolve the whole sample matrix. Because determining the (bio-)availability of elements is an important question in environmental studies, acid leaching is a popular choice here. Typical acids for leaching of species from the sample matrix are HCl and HNO3 – preferably a (diluted) mixture (3:1, aqua regia or 1:3, inverse aqua regia).
Why use microwave digestion for sample preparation?
Microwaves are electromagnetic waves in the range of 300 MHz (0.3 GHz) to 300 GHz, with a typical frequency of 2.450 MHz for both domestic microwave ovens and laboratory equipment (see Figure 2).[4]
Microwaves are particularly well-suited for sample preparation tasks. We explain why below.
Fast heating rates
With microwaves, electromagnetic energy is converted into heat energy highly efficient, which results in extremely fast heating rates. These heating rates are not reproducible with conventional heating. With conventional heating, the heat comes from the outside and goes into the reaction mixture by convection currents (resulting in a very hot vessel wall). In contrast, microwaves pass through the (almost) microwave-transparent vessel wall and heat the reaction mixture on a molecular basis – by direct interaction with the molecules (Figure 3).
Instant turn on and turn off
While microwave radiation can be instantly turned on and off, conventional heating cannot. This means the vessels continue to be heated even after the heating source has been turned off. This capability for instant turn on and turn off lets the instruments put in power when needed and to shut off heating immediately when required (i.e., in cases of temperature overshoots or too fast heating).
No contact to heating core required
Due to the way microwave heating works (see Figure 2), no direct contact to a heating core is necessary. This lets you heat different vessel sizes, shapes, and amounts with the same microwave system. Besides that, parallel processing is state of the art in microwave digestion, which means that several samples and blanks can be processed simultaneously under similar conditions.
High temperatures and pressures can be reached
Modern microwave sample preparation systems let you reach temperatures up to 300 °C and pressures up to 200 bar due to their safe and corrosion-resistant design. This lets you conduct successful digestions that could not be done at room temperature or when only heating the acid mixtures at their boiling point.
What parameters are important in microwave digestion?
Temperature
The most important parameter for acid digestion and acid leaching is the temperature. High temperature has two important functions. First, to speed up the digestion reaction. Second, to improve the digestion quality due to the increased oxidation potential of the used acid mixture.
Time
According to the Arrhenius equation,[5] an increase in temperature will lead to a decrease in reaction time (Figure 4). There are multiple reasons as to why microwaves shorten digestion times, including their very fast heating effect, their ability to be turned on and off immediately, and their optimized workflow.
Digestion quality
A higher temperature also increases the oxidation potential of the acids, which has a positive effect on the digestion quality because a temperature increase leads to a decrease in the amount of residual carbon. The residual carbon content is a good parameter to characterize the digestion performance since low residual carbon values result in less interference during the elemental analysis. Figure 5 shows the residual carbon content in dependence of the digestion temperature: The higher the temperature, the lower the residual carbon content is.
This can also be seen in Figure 6: Lubrication oil was digested at different temperatures using the same digestion matrix and digestion time. While the sample was digested completely (resulting in a clear solution) at 260 °C,170 °C was not enough for full digestion (high amounts of residual carbon are visible from the color of the digestion matrix).
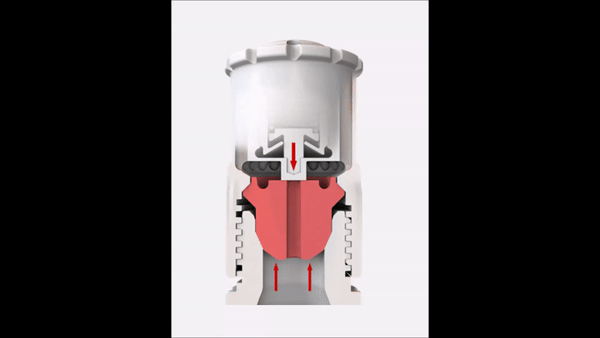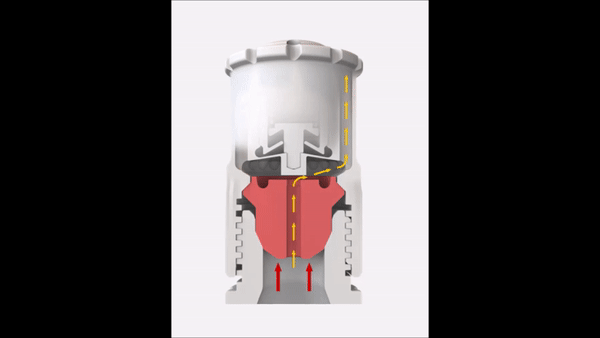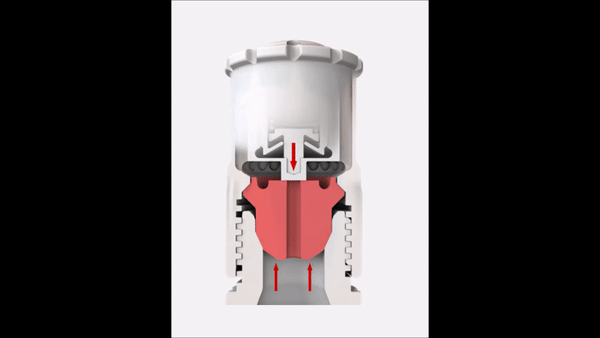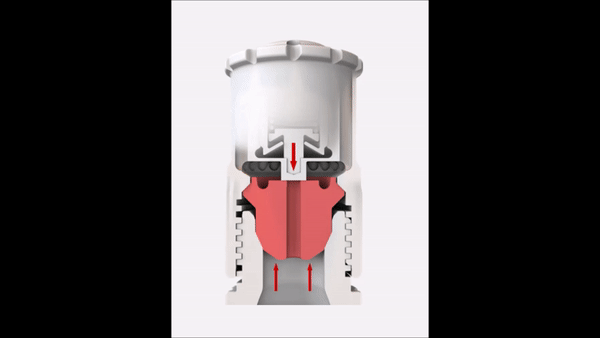
Sample weight
In order to reach high temperatures beyond the boiling point of the used acids, you have to seal the digestion vessels. This results in elevated pressures because of the increased vapor pressure of the heated acids. In addition to the vapor pressure, the digestion reaction also contributes to the built-up pressure inside the vessel. Depending on the sample material that has to be digested, more or less gaseous products are formed during the digestion according to the equation in Figure 7.

Figure 7: General reaction scheme of an organic sample being digested with nitric acid. Total pressure in the sealed vessel = vapor pressure of the acid + reaction pressure from the sample
Since the reaction pressure correlates with the amount of the digested material, the sample weight is an important factor in microwave digestion. High sample weights limit the achievable temperature in completely closed vessel systems. This short video explains the correlation between temperature and sample weight in such a system.
In order to overcome this temperature limitation with higher sample weights, SmartVent technology is used in some microwave digestion systems. This technology lets you safely release reaction gases during digestion so you can maintain higher temperature levels for better digestion quality.
Instruments for microwave digestion
The cavity is the part of a microwave system where the microwave digestion vessel is placed and irradiated/heated. This is the central part of each microwave digestion system. Depending on the instrument design, there are different irradiation modes.
Monomode microwave systems
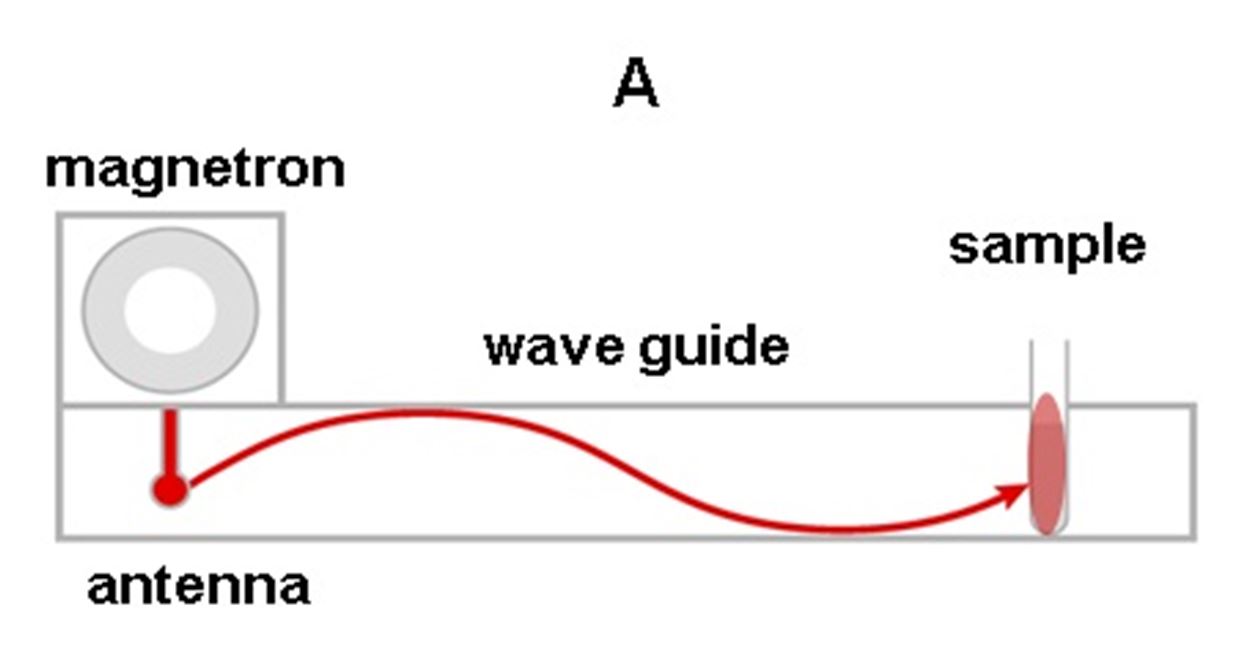
In these systems, microwave energy is created by a single magnetron and directed through a waveguide[6] to the reaction mixture (see Figure 8). This results in a “standing wave,” and the reaction vessel is located in a hot spot where efficient heating of small volumes is possible. Monomode microwave reactors have a space-saving design, but they can only digest one sample at a time.
Multimode microwave systems
In multimode microwave systems, one or two magnetrons are used to create microwave irradiation. The irradiation is directed into the cavity through a waveguide and distributed by a mode stirrer (see Figure 9). Microwaves are reflected from the walls and thus interact with the reaction mixture in a random manner. Additional rotation of the reaction vessel in the cavity prevents temperature inhomogeneity and formation of hot spots.
This mode lets you heat larger volumes (>20 mL) of reaction mixtures and digest several samples simultaneously (up to 64 samples).
Directed Multimode Cavity (DMC)
The DMC combines the benefits of monomode and multimode reactors. Like in a monomode system, the microwaves are directed to the samples, which provides highly efficient heating in a system with a small footprint. But, like in a multimode system, up to 12 samples can be digested in a single run (see Figure 10).
Pressurized Digestion Cavity (PDC)
In these systems, microwave energy is created by a single magnetron and directed to the PDC (Pressurized Digestion Cavity, Figure 11). A PTFE liner, filled with a load solution, is placed in the cavity and is heated with the reaction media. Heating a load solution instead of a specific vessel ensures an optimized temperature distribution and compensates exothermic reactions. The digestion temperature is permanently controlled by a temperature sensor on the bottom of the PDC. This lets you use one (but up to 28) thin-walled digestion vial (made of quartz, glass, or fluoropolymer) closed with simple plug-on caps without a required minimum filling volume. “Sealing” of the vials works with pressurized inert gas that is filled in the cavity prior to heating. This prevents boiling and evaporation of the reagent mixture.
These systems have larger footprints. But they are commonly used systems for microwave digestion because of their flexibility when it comes to different sample types, sample weights, digestion volumes, and matrices.
Helpful links
- Find available systems for microwave digestion.
- Browse digestion methods for microwave digestion.
References
- Cammann, K. (2010). Instrumentelle Analytische Chemie. Spektrum Akademischer Verlag, Heidelberg.
- Bulska, E., Matusiewicz, H. (2018). Inorganic Trace Analytics: Trace Element Analysis and Speciation, De Gruyter, Berlin.
- Harris, D. C. (2014). Lehrbuch der Quantitativen Analyse. 8th Edition, Vieweg+Teubner Verlag, Wiesbaden, 796.
- Wikipedia, (2023). https://en.wikipedia.org/wiki/Microwave
- Meyer, H., and E. Riedel. 2018. Allgemeine und Anorganische Chemie. 12th Edition, De Gruyter, Berlin, 180.
- Techopedia, (2023). https://www.techopedia.com/definition/722/waveguide