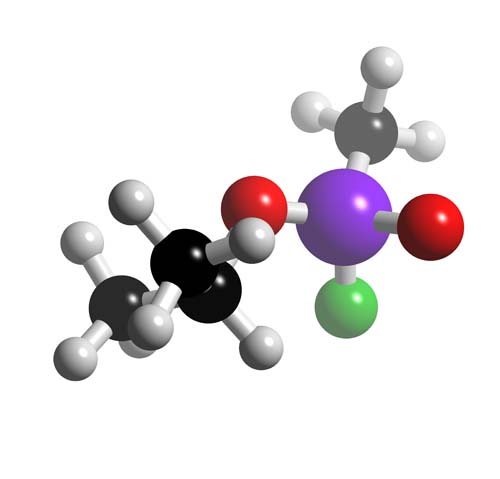
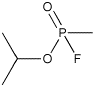
Sarin (Molecule of the Month for October 2001)
A Nerve Gas
Nerve agents such as Sarin have been the chemical weapons of choice since World War II.
Sarin was first produced by German scientists in 1938 ; although it was not used during the war, many have speculated that it could have given the Germans a decisive advantage.
Sarin is a colourless, odourless gas with a lethal dose of just 0.5mg for an adult human (or 0.01mg/kg of body weight). It is a member of the organophosphate family which were previously used as pesticides before their effects were fully known. Sarin possess the typical organophosphate characteristics of stability, high toxicity, and is an easily dispersed agent that acts extremely quickly when absorbed through the skin or inhaled. Although recently superseded by more deadly and more persistent compounds (Sarin is classified as non persistent) such as VX Gas, Sarin remains a highly dangerous and effective part of mankind's destructive arsenal.
Like other nerve agents, Sarin functions by competitive inhibition of the enzyme acetyl cholinesterase. This enzyme is found at synapses and nerve endings where it breaks hydrolyses the neurotransmitter Acetyl choline so that the nerve impulse is only transmitted once as required. When the enzyme is inhibited acetyl choline accumulates at nerve endings. This has various unpleasant effects leading to paralysis (where accumulation occurs at motor neurones) and eventually death by means such as asphyxiation.
The final stage of the synthesis usually takes place while the missile or other delivery vessel is in flight because it is safer to store the reagents which are far less dangerous than Sarin itself.
Current decontamination systems:
The hydrolysis reaction is rapid and used for the decontamination of affected areas:
- Solids, powders and solutions containing various types of bleach ( NaOCl- or Ca(OCl-)2 )
- DS2 ( 2% NaOH, 70% diethylenetriamine, 28% ethylene glycol monomethyl ether )
- Towlettes moistened with NaOH dissolved in water, phenol, ethanol and ammonia
Formal Chemical Name (IUPAC)
Isopropylmethanefluorophosphonate
Update by Karl Harrison
(Molecule of the Month for
October 2001
)
 All the images on this web site are are made available with a Creative Commons Attribution license and so can be used as long as the attribution © Karl Harrison 3DChem.com is written with the image. High resolution images and illustrations are available on request.
All the images on this web site are are made available with a Creative Commons Attribution license and so can be used as long as the attribution © Karl Harrison 3DChem.com is written with the image. High resolution images and illustrations are available on request.