Feldspar
The name feldspar derives from the German Feldspat, a compound of the words Feld (field) and Spat (flake). Spat had long been used as the word for "a rock easily cleaved into flakes". Feldspat was introduced in the 18th century as a more specific term, referring perhaps to its common occurrence in rocks found in fields (Urban Brückmann, 1783). The change from Spat to -spar was influenced by the English word spar, meaning a non-opaque mineral with good cleavage. Feldspar is the most abundant mineral group in the Earth�s crust. There are more feldspars (60%) than all the other minerals combined in the outer (13-17) km of the crust.Feldspars include three compositional endmembers: K-feldspar (KAlSi3O8), albite (NaAlSi3O8) and anorthite (CaAl2Si2O8) Fig.1.
Alkali feldspars are compositionally between Or and Ab endmembers. Plagioclase feldspars are between Ab and An. There are no feldspars that are intermediate in composition between K (Or) and Ca (An) endmembers because these ions have different ionic radii and charges which would make the structure unstable. Some uncommon feldspars contain barium in the crystal structure. They are celsian (BaAl2Si2O8) and hyalophane (compositionally between K-feldspar and celsian). They are also structurally similar to K-feldspars. These minerals are volumetrically very restricted, nowhere near as common as other feldspars.
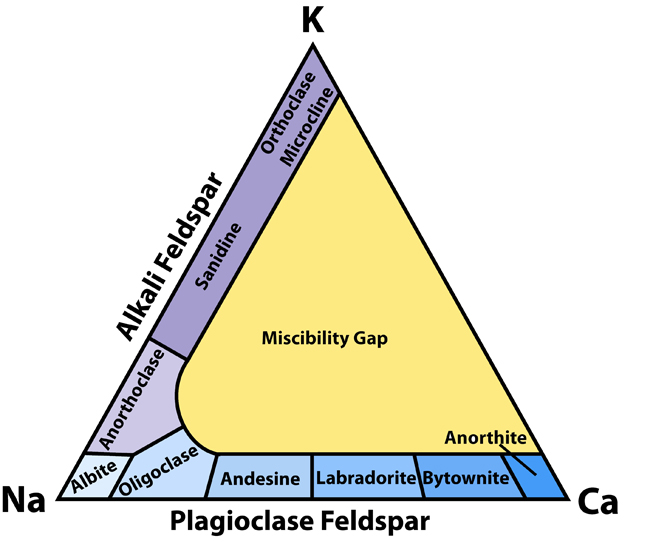
Fig.1: Feldspar classification. This diagram shows how feldspar minerals are classified on the basis of their chemical composition. The sequence of minerals along the base of the triangle represents the solid solution series of plagioclase between albite and anorthite.
Plagioclase
Plagioclase feldspars are the most common feldspar minerals because calcium is somewhat more common in the crust than potassium (3.6 and 2.8 percent of the crust, respectively). Plagioclase feldspars form a continuous solid solution between Ab and An endmembers at high temperature, but the replacement of ions needs to be coupled because of the charge difference between Na+ and Ca2+. The charge balance in maintained by substituting Al3+ for Si4+. The amount of potassium that may enter the lattice is limited because of large difference in ionic radii.The plagioclase series is arbitrarily divided into six minerals or compositional ranges: albite (Ab90 - Ab100), oligoclase (Ab70 - Ab90), andesine (Ab50 - Ab70), labradorite (Ab30 - Ab50), bytownite (Ab10 - Ab30), and anorthite (Ab0 - Ab10). These boundaries have no structural significance. Their use is justified because plagioclase is very common mineral and occurs in a wide variety of rocks and the composition of plagioclase is rather predictable. For example, it is common to find sodic plagioclase (oligoclase) in granite, more calcium-rich varieties (labradorite) in mafic rocks like gabbro, and intermediate andesine in intermediate igneous rocks like andesite.
Alkali feldspar
Unlike plagioclase feldspars, alkali feldspars are divided into separate minerals not only based on their chemistry, but more importantly, based on optical properties determined by a petrographic microscope. Most alkali feldspars are compositionally closer to Or end-member which explains why we often refer to them as K-feldspars (potassium feldspars).Microcline is a triclinic K-feldspar and has a characteristic cross-hatched tartan pattern of twinning seen with a petrographic microscope.
Orthoclase and sanidine are both optically monoclinic K-feldspars. Sanidine occurs in volcanic and subvolcanic rocks. Orthoclase occurs in plutonic rocks.
Adularia is a K-feldspar produced by low-temperature hydrothermal (veins) or authigenic processes. It is structurally similar to microcline but without the cross-hatched twinning.
Anorthoclase is the only alkali feldspar that is not K-feldspar. It is sodic in composition, optically triclinic and characterized by a twinning similar to microcline but on a smaller scale.
The structural state which is the basis of the alkali feldspar classification, is dependent on the temperature of crystallization and subsequent cooling history. Volcanic feldspars tend to retain their high temperature ordering because the cooling is very rapid (sanidine). Low-temperature feldspars either crystallized at lower temperatures (adularia) or had ample amount of time to cool down (microcline).
Bibliography
• Cox et al. (1979): The Interpretation of Igneous Rocks, George Allen and Unwin, London.
• Howie, R. A., Zussman, J., & Deer, W. (1992). An introduction to the rock-forming minerals (p. 696). Longman.
• Le Maitre, R. W., Streckeisen, A., Zanettin, B., Le Bas, M. J., Bonin, B., Bateman, P., & Lameyre, J. (2002). Igneous rocks. A classification and glossary of terms, 2. Cambridge University Press.
• Middlemost, E. A. (1986). Magmas and magmatic rocks: an introduction to igneous petrology.
• Shelley, D. (1993). Igneous and metamorphic rocks under the microscope: classification, textures, microstructures and mineral preferred-orientations.
• Vernon, R. H. & Clarke, G. L. (2008): Principles of Metamorphic Petrology. Cambridge University Press.

