Abstract
Objectives. We evaluated malodor and air pollutants near industrial hog operations as environmental stressors and negative mood triggers.
Methods. We collected data from 101 nonsmoking adults in 16 neighborhoods within 1.5 miles of at least 1 industrial hog operation in eastern North Carolina. Participants rated malodor intensity, stress, and mood for 2 weeks while air pollutants were monitored.
Results. Reported malodor was associated with stress and 4 mood states; odds ratios (ORs) for a 1-unit change on the 0-to-8 odor scale ranged from 1.31 (95% confidence interval [CI] = 1.16, 1.50) to 1.81 (95% CI = 1.63, 2.00). ORs for stress and feeling nervous or anxious were 1.18 (95% CI = 1.08, 1.30) and 1.12 (95% CI = 1.03, 1.22), respectively, for a 1 ppb change in hydrogen sulfide and 1.06 (95% CI = 1.00, 1.11) and 1.10 (95% CI = 1.03, 1.17), respectively, for a 1 μg/m3 change in semivolatile particulate matter less than 10 μm in aerodynamic diameter (PM10).
Conclusions. Hog odor, hydrogen sulfide, and semivolatile PM10 are related to stress and negative mood in disproportionately low-income communities near industrial hog operations in eastern North Carolina. Malodor should be considered in studies of health impacts of environmental injustice.
Odor, noise, heat, and crowding are environmental stressors1 that may affect physical and mental health. Malodor is reported in neighborhoods near hazardous waste facilities, petroleum refineries, certain industrial facilities, and confined animal feeding operations; people in these areas may report sensations of irritation, respiratory problems and other physical health symptoms, interference with activities of daily living, and concerns about chronic diseases and property values.1–37 Because polluting facilities are disproportionately located in low-income communities and communities of color,38,39 malodor is an important aspect of environmental injustice that threatens physical, mental, and social well-being.40
Several studies have evaluated relationships among malodor, negative mood, and reduced quality of life in neighbors of industrial hog operations. Schiffman et al.26 found that a small sample of neighbors of industrial hog operations reported more tension, depression, anger, fatigue, and confusion, and less vigor, compared with an unexposed rural sample. Bullers4 found higher mean scores on a short form of the Center for Epidemiologic Studies Depression Scale (CES-D) in neighbors of industrial hog operations than in control participants (2.24 vs 1.84). Wing and Wolf36 assessed effects on quality of life, determined by asking how often neighbors of hog operations could open windows or go outside during nice weather. By that metric, neighbors reported greatly reduced quality of life relative to other demographically comparable rural residents.
The Community Health Effects of Industrial Hog Operations (CHEIHO) study was a collaborative community-based participatory research project conducted in the predominantly low-income African American communities of rural eastern North Carolina where industrial hog operations are disproportionately located.35 The purpose of this study was to evaluate longitudinal relationships among malodor, airborne emissions, stress, and negative mood. We hypothesized that malodor from industrial hog operations is an environmental stressor that may also negatively affect mood.
METHODS
We have previously described the CHEIHO study, including details of its community-based design and its links to education and organizing for environmental justice.41 Research on health effects in neighbors of industrial hog operations is community-based at its origin. Community-based organizations brought the issue to the attention of researchers at the School of Public Health at the University of North Carolina and have continued as partners in all research that has been conducted. In the CHEIHO study, members of community-based organizations participated as advisors in the study design and design of study instruments. They were integrally involved in the recruitment and training of study participants. Indeed, community organizers were essential to the recruitment and retention of study participants in predominantly African American communities with historic distrust of researchers and research institutions.42
Study Participants
Eligible participants in the CHEIHO study were nonsmoking adults who lived within 1.5 miles of at least 1 industrial hog operation and were willing to collect data twice daily for approximately 2 weeks. Between September 2003 and September 2005, participants collected data on odor, stress, mood, physical health symptoms, blood pressure, immune function, and lung function; outcomes analyzed in this study are described in more detail in the paragraphs that follow.
At a central location in each neighborhood, research staff set up a monitoring trailer to collect data on hydrogen sulfide (H2S; MDA Scientific Single Point Monitor, Honeywell Analytics Inc North America, Lincolnshire, IL), particulate matter less than 10 μm in aerodynamic diameter (PM10) and semivolatile PM10 (Tapered Element Oscillating Microbalance Series 1400a Ambient Particulate Monitor with a Series 8500 Filter Dynamics Measurement System, Thermo Fisher Scientific, Waltham, MA), and weather (Vantage Pro Weather Station, Davis Instruments, Hayward, CA, and Young Model 05103VM-42 Wind Monitor, R. M. Young Company, Traverse City, MI).
Selection of the particular pollutants to be monitored was based on previous work that has documented emissions of both H2S (a product of the anaerobic decomposition of hog waste) and particulate matter from feed, dried feces, skin cells, hair, and bioaerosols, at confinement buildings and waste lagoons.6,43 Furthermore, we found that H2S and PM10 were related to reported malodor in the CHEIHO study; H2S was associated with reported malodor in models that adjusted for the study's longitudinal design, as was PM10 during times when wind speed was greater than 6.75 miles per hour.44
The average distance from the monitoring platform to the nearest industrial hog operation in each neighborhood was 0.51 miles; the minimum distance to the nearest industrial hog operation was 0.20 miles and the maximum distance to the nearest industrial hog operation was 1.42 miles. In 2 of the 16 neighborhoods, the platform was located within 2 miles of 1 industrial hog operation; in the other 14 neighborhoods, however, the platform was located within 2 miles of at least 3 industrial hog operations (maximum of 16 industrial hog operations). We therefore calculated, for each neighborhood, the average distance between the platform and the industrial hog operations within 2 miles of the monitoring platform. The average distance across all neighborhoods was 1.10 miles, with a range by neighborhood from 0.56 miles to 1.50 miles. In contrast, the average distance between participant households and the monitoring platform across 15 of the 16 neighborhoods was 0.20 miles, with a range by neighborhood from 0.03 miles to 0.36 miles.
In 1 neighborhood, the average distance between participant households and the platform was 0.95 miles. In this and 3 other neighborhoods where participant homes were more geographically dispersed, we deployed additional H2S monitors at homes farthest from the platform. All of the data on particulate matter, however, were collected at the platform and assigned to all participants in the neighborhood.
Participants attended a 3-hour training session during which they learned to complete the required data collection activities. They selected a morning time and an evening time at which they would collect data (for example, 6:00 AM and 6:00 PM). In addition, participants completed an assessment of coping style using the John Henryism Active Coping scale45,46 and an assessment of threshold odor sensitivity using butanol standards.47
At the preselected, twice-daily times, participants spent 10 minutes outdoors at home and then returned indoors to rate any odor present during that 10-minute period on a 9-point scale ranging from 0 (no odor) to 8 (very strong odor). Hourly average H2S, PM10, and semivolatile PM10 values were calculated for the hour immediately preceding the odor rating. Following the odor rating, they responded to 5 mood state questions: “How do you feel now: (a) stressed or annoyed?, (b) nervous or anxious?, (c) gloomy, blue, or unhappy?, (d) angry, grouchy, or bad-tempered?, (e) confused or unable to concentrate?” They rated these mood questions on a 9-point scale ranging from 0 (not at all) to 8 (extremely). The “stressed or annoyed?” question was an ad-hoc single-item measure,48,49 and the remaining 4 questions came from the Profile of Mood States instrument,26,50 specifically, from the Tension–Anxiety, Depression–Dejection, Anger–Hostility, and Confusion–Bewilderment subscales. (The Fatigue–Inertia and Vigor–Activity subscales were not used.)
Statistical Analyses
We used logistic mixed models to evaluate malodor, H2S, PM10, and semivolatile PM10 as predictors of reported stress and negative mood (NLMIXED procedure in SAS version 9.1.3, Cary, NC). We used 2-level (within person and between person) mixed models to take into account the correlated structure of longitudinal data for individuals. The stress and mood variables were recoded as binary; for stressed or annoyed and nervous or anxious, 0 and 1 on the original scale were coded as 0 and 2 to 8 on the original scale were coded as 1. For the remaining 3 mood variables, 0 on the original scale was also coded as 0 and 1 to 8 on the original scale were coded as 1. These coding decisions were based on the distribution of the data such that approximately 90% of the records for each outcome variable were coded as 0 and approximately 10% were coded as 1. We included all predictor variables as linear terms. We conducted all analyses with records for which the ratings of malodor, stress, and mood, and the airborne emissions data, were not missing.
Random intercepts were included in the mixed logistic models to capture the variation between participants in baseline (average) levels of stress and negative mood. Models included the following time-dependent covariates: time of day (morning vs evening), study day (1 to ≥ 14), and study week (first vs second). For analyses of odor as a predictor of stress and mood, the models also included whether participants reported a cold, flu, or stomach virus at any time during data collection (yes or no). We hypothesized that illness could affect a participant's ability to smell or perception of odor and negative mood. We did not consider time-independent confounders, such as age or gender, because their relationship with exposure and outcome did not vary over time. A sample logistic mixed model follows.
Level 1 (time, within person):
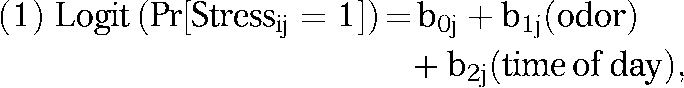 |
where Pr[Stressij = 1] is the probability that stress reported by person j at timepoint i equaled 1, b0j is the person-specific intercept, b1j is the effect of the time-dependent odor rating, and b2j is the effect of time of day (morning vs evening).
Level 2 (between person):
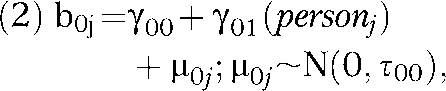 |
where b0j is the person-specific intercept, γ00 is the mean of the person-specific intercepts (i.e., fixed intercept), γ01(personj) is the contribution to the overall mean from person j, and μ0j is the residual between-person variation in the intercept.
We also evaluated several potential modifiers. For analyses of H2S as a predictor of stress and negative mood, we considered modification by wind speed (low [≤ 0.57 mph], medium [0.58 mph–6.75 mph], and high [> 6.75 mph]) because of previous work that suggested modification of the relationship between H2S and reported malodor by wind speed.44 Based on previous research,3,29,30,37 we considered age, dichotomized at the median (≤ 53.7 years vs > 53.7 years), and coping style, dichotomized at the median, (John Henryism Active Coping scale score < 52 vs ≥ 52)46,47 as potential modifiers of any association between reported odor and stress. We also considered threshold odor sensitivity (low or moderate [< 320 ppm] vs high [≥ 320 ppm]) as a potential modifier of the relationships between odor, stress, and mood to evaluate whether more-sensitive individuals responded differently than less-sensitive ones.
RESULTS
There were 2895 records from 101 individuals in 16 neighborhoods. Complete data on reported odor, stress, and mood were available for 2666 records. Of the 2666 records with complete odor, stress, and mood data from study participants, 78 records were missing data on H2S and 741 records were missing data on PM10 because of monitoring equipment malfunction.
Demographics
Table 1 presents demographic information about study participants. The median age was 53.7 years; age ranged from 19.2 years to 89.5 years. Approximately two thirds of the participants were female, and approximately 85% were African American. Seventy-five percent of participants reported that they grew up around livestock. Six neighborhoods were within 2 miles of 1 to 4 industrial hog operations, 4 were within 2 miles of 5 to 9 industrial hog operations, and 6 were within 2 miles of 10 or more industrial hog operations. Average H2S values in the 16 neighborhoods ranged from less than 0.01 ppb to 1.5 ppb, and the highest measured H2S values ranged from 2 ppb to 90 ppb. Average PM10 values ranged from 10.8 μg per cubic meter (μg/m3) to 28.7 μg/m3, and average semivolatile PM10 values ranged from −3.2 μg/m3 (negative values occurred because of measurement imprecision at very low concentrations) to 9.2 μg/m3.44
TABLE 1.
Participant Characteristics: Community Health Effects of Industrial Hog Operations Study, Eastern North Carolina, 2003–2005
| No. of Records | No. of Participants | |
| Age | ||
| > 53.7 y | 1377 | 50 |
| ≤ 53.7 y | 1289 | 51 |
| Gender | ||
| Female | 1737 | 66 |
| Male | 929 | 35 |
| Race | ||
| Black | 2167 | 85 |
| Non-Blacka | 499 | 16 |
| Grew up around livestock | ||
| Yes | 1998 | 76 |
| No | 591 | 22 |
| Missing | 77 | 3 |
| Total | 2666 | 101 |
Fifteen White participants and 1 Latino participant.
The distribution of twice-daily odor ratings during the preselected 10-minute exposure times is presented in Table 2. Of the 2666 odor ratings recorded after participants spent 10 minutes outdoors, approximately 50% equaled zero. An additional 30% were low (a rating of 1 or 2) on the 9-point scale. Approximately 20% were 3 or higher, and 1% of the data were in each of the 2 highest categories. Most of the ratings of stress and mood state also equaled zero. For “stressed or annoyed,” 81% of reports were zero; 87% were zero for “nervous or anxious,” 88% for “gloomy, blue, or unhappy,” 93% for “angry, grouchy, or bad-tempered,” and 95% for “confused or unable to concentrate” (Table 2).
TABLE 2.
Number and Percentage of Records and Number of Participants in Each Category of the Odor, Stress, and Mood Variable Ratings: Community Health Effects of Industrial Hog Operations Study, Eastern North Carolina, 2003–2005
| Twice-Daily Odor Rating |
Stressed or Annoyed |
Nervous or Anxious |
Gloomy, Blue, or Unhappy |
Angry, Grouchy, or Bad-Tempered |
Confused or Unable to Concentrate |
|||||||
| Rating | No. of Records (%) | No. of Participants | No. of Records (%) | No. of Participants | No. of Records (%) | No. of Participants | No. of Records (%) | No. of Participants | No. of Records (%) | No. of Participants | No. of Records (%) | No. of Participants |
| 0 | 1374 (51.5) | 88 | 2162 (81.1) | 98 | 2314 (86.8) | 100 | 2337 (87.7) | 98 | 2479 (93.0) | 99 | 2529 (94.9) | 100 |
| 1 | 472 (17.7) | 82 | 290 (10.9) | 60 | 217 (8.1) | 40 | 198 (7.4) | 44 | 109 (4.1) | 40 | 96 (3.6) | 24 |
| 2 | 273 (10.2) | 72 | 95 (3.6) | 39 | 80 (3.0) | 24 | 42 (1.6) | 20 | 22 (0.8) | 11 | 20 (0.8) | 9 |
| 3 | 196 (7.4) | 68 | 50 (1.9) | 20 | 34 (1.3) | 12 | 45 (1.7) | 13 | 10 (0.4) | 7 | 10 (0.4) | 4 |
| 4 | 123 (4.6) | 47 | 14 (0.5) | 10 | 10 (0.4) | 3 | 12 (0.5) | 6 | 6 (0.2) | 5 | 7 (0.3) | 2 |
| 5 | 73 (2.7) | 39 | 22 (0.8) | 13 | 8 (0.3) | 6 | 13 (0.5) | 6 | 17 (0.6) | 9 | 3 (0.1) | 3 |
| 6 | 108 (4.1) | 40 | 19 (0.7) | 10 | 1 (< 0.1) | 1 | 8 (0.3) | 4 | 10 (0.4) | 4 | 1 (< 0.1) | 1 |
| 7 | 22 (0.8) | 12 | 6 (0.2) | 4 | 1 (< 0.1) | 1 | 6 (0.2) | 3 | 5 (0.2) | 3 | 0 (0.0) | 0 |
| 8 | 25 (0.9) | 12 | 8 (0.3) | 6 | 1 (< 0.1) | 1 | 5 (0.2) | 3 | 8 (0.3) | 3 | 0 (0.0) | 0 |
| Total | 2666 (100.0) | 101 | 2666 (100.0) | 101 | 2666 (100.0) | 101 | 2666 (100.0) | 101 | 2666 (100.0) | 101 | 2666 (100.0) | 101 |
Mixed Models
Table 3 presents parameter estimates, standard errors, t values, odds ratios (ORs), and 95% confidence intervals (CIs) for H2S, PM10, semivolatile PM10, and reported malodor as predictors of binary stress and negative mood. Variables considered as time-dependent confounders produced little change in the magnitude of the parameter estimates for the independent variables. However, we adjusted all models for time of day (morning vs evening) because time is an important predictor of odor. Reporting stress or annoyance was strongly associated with increasing levels of H2S; the OR for a 1 ppb change in H2S was 1.18 (95%CI = 1.08, 1.30). Hydrogen sulfide was also strongly associated with feeling nervous or anxious (OR = 1.12; 95% CI = 1.03, 1.22). Hydrogen sulfide did not appear to be associated with the other 3 mood state variables, and wind speed did not modify any of the relationships between H2S and stress or mood.
TABLE 3.
Logistic Mixed Model Results for Associations Between Hydrogen Sulfide, PM10, Semivolatile PM10, Odor, Stress, and Negative Mood: Community Health Effects of Industrial Hog Operations Study, Eastern North Carolina, 2003–2005
| Main Exposure and Outcome Variable | b | SE | t | OR (95% CI) |
| Hydrogen sulfide (ppb) | ||||
| Stressed or annoyed | 0.17 | 0.048 | 3.54 | 1.18 (1.08, 1.30) |
| Nervous or anxious | 0.11 | 0.044 | 2.55 | 1.12 (1.03, 1.22) |
| Gloomy, blue, or unhappy | 0.012 | 0.063 | 0.18 | 1.01 (0.89, 1.15) |
| Angry, grouchy, or bad-tempered | 0.039 | 0.047 | 0.84 | 1.04 (0.95, 1.14) |
| Confused or unable to concentrate | −0.074 | 0.12 | −0.63 | 0.93 (0.73, 1.17) |
| PM10 (μg/m3) | ||||
| Stressed or annoyed | 0.00065 | 0.0051 | 0.13 | 1.00 (0.99, 1.01) |
| Nervous or anxious | 0.0029 | 0.0052 | 0.57 | 1.00 (0.99, 1.01) |
| Gloomy, blue, or unhappy | 0.012 | 0.010 | 1.11 | 1.01 (0.99, 1.03) |
| Angry, grouchy, or bad-tempered | 0.0035 | 0.0057 | 0.61 | 1.00 (0.99, 1.01) |
| Confused or unable to concentrate | 0.010 | 0.0070 | 1.43 | 1.01 (1.00, 1.02) |
| Semivolatile PM10 (μg/m3) | ||||
| Stressed or annoyed | 0.055 | 0.025 | 2.15 | 1.06 (1.00, 1.11) |
| Nervous or anxious | 0.095 | 0.033 | 2.91 | 1.10 (1.03, 1.17) |
| Gloomy, blue, or unhappy | 0.058 | 0.043 | 1.35 | 1.06 (0.97, 1.16) |
| Angry, grouchy, or bad-tempered | 0.027 | 0.026 | 1.05 | 1.03 (0.98, 1.08) |
| Confused or unable to concentrate | 0.043 | 0.036 | 1.22 | 1.04 (0.97, 1.12) |
| Twice daily odor rating (0–8) | ||||
| Stressed or annoyed | 0.59 | 0.051 | 11.50 | 1.81 (1.63, 2.00) |
| Nervous or anxious | 0.47 | 0.064 | 7.37 | 1.60 (1.41, 1.81) |
| Gloomy, blue, or unhappy | 0.36 | 0.067 | 5.35 | 1.43 (1.25, 1.63) |
| Angry, grouchy, or bad-tempered | 0.42 | 0.055 | 7.70 | 1.52 (1.37, 1.70) |
| Confused or unable to concentrate | 0.27 | 0.065 | 4.20 | 1.31 (1.16, 1.50) |
Note. OR = odds radio; CI = confidence interval; PM10 = particulate matter less than 10 μm in aerodynamic diameter. Adjusted for time of day, morning versus evening.
We found that PM10 did not appear to be associated with stress or negative mood, with the exception of a marginal association with feeling confused or unable to concentrate (Table 3). Semivolatile PM10 was most strongly associated with feeling stressed or annoyed and nervous or anxious. Associated ORs for a 1 μg/m3 increase in semivolatile PM10 were small (1.06 and 1.10, respectively), though ORs associated with a 10 μg/m3 increase, for example, were 1.73 and 2.59, respectively. Semivolatile PM10 appeared to be only marginally associated with feeling gloomy, angry, or confused or unable to concentrate.
Table 3 also presents parameter estimates, standard errors, t values, ORs, and 95% CIs for reported malodor as a predictor of binary stress and negative mood. All parameter estimates were large relative to their standard errors. The ratio of the odds of reporting stress for a 1-unit increase in reported odor on a 0-to-8 scale was 1.81 (95% CI = 1.63, 2.00). Consequently, a 4-unit change on the odor scale (from odor = 0 to odor = 4, for example) yielded an OR of 10.6. Odds ratios for feeling nervous, gloomy, angry, and unable to concentrate, associated with a 1-unit change in odor, were 1.60 (95% CI = 1.41, 1.81); 1.43 (95% CI = 1.25, 1.63); 1.52 (95% CI = 1.37, 1.70) and 1.31 (95% CI = 1.16, 1.50), respectively.
Coping, but not age, appeared to modify the relationship between reported odor and stress. The parameter estimate for participants who scored below the median on the John Henryism Active Coping scale was 0.45 (standard error [SE] = 0.07), whereas the parameter estimate for participants who scored above the median was 0.73 (SE = 0.08). Threshold odor sensitivity did not appear to modify the associations between reported malodor and stress or negative mood.
DISCUSSION
We used a longitudinal design to evaluate relationships between malodor from industrial hog operations, H2S, PM10, semivolatile PM10, and the stress and negative mood reported by neighboring residents. We found that ratings of feeling stressed or annoyed, nervous or anxious, gloomy or unhappy, angry or grouchy, and confused or unable to concentrate increased with ratings of malodor. Of the 5 outcome variables, odor was most strongly related to feeling stressed or annoyed. Active coping appeared to modify the relationship between odor and stress or annoyance, with those with higher John Henryism scores more affected by malodor. Hydrogen sulfide appeared to be associated with feeling stressed or annoyed and nervous or anxious but not with the other 3 mood variables. We found that PM10 was not associated with the outcome variables, with the exception of a marginal association with feeling confused or unable to concentrate. Semivolatile PM10, however, appeared to be associated with feeling stressed or annoyed and nervous or anxious and only marginally associated with the remaining 3 mood variables.
Though we are not aware of other work that has sought to link airborne emissions to reported stress and negative mood, there is a consistent literature documenting the effect of malodor on annoyance, both in laboratories1,37,51–53 and other settings.3,29,30 Several authors have also considered coping style as a potential effect modifier.1,3,29,30,37 In field studies of annoyance response to industrial odors, people with higher scores for problem-oriented coping, or action-oriented coping, tended to report more annoyance following odor exposure than did people with lower scores.3,29,30,37 In a laboratory study, however, Asmus and Bell did not find coping style to be an effect modifier.1
We found a stronger relationship between odor and stress in participants with high scores on the John Henryism Active Coping scale. Our findings are consistent with odor studies by Steinheider and Winneke,29 Winneke et al.,37 Sucker et al.,30 and Both et al.3 The John Henryism Active Coping scale was developed by Sherman James in studies conducted among African Americans in eastern North Carolina46 and, therefore, may be especially appropriate in the context of the present investigation. It measured “the degree to which [Black Americans] felt they could control their environment through hard work and determination.”46(p259) James hypothesized a poorer health outcome (higher blood pressure) in men who scored high on the scale but lacked the resources to control their environments.46 Consistent with our a priori hypothesis, it appears that study participants who perceived that they had more control over their environment found an unpredictable and uncontrollable malodor more stressful than those who perceived they had less control.
Strengths and Limitations
The longitudinal design was a particular strength of this research. There were approximately 28 repeated measures for each participant. In the analyses, each participant served as her or his own control. Perceptions of stress and adverse mood vary between people, and we were able to statistically model the between-person variation in such perceptions. Physical measures of pollution are an additional strength of this research; previous studies have relied entirely on self-reported measures of exposure and outcome. We did, however, measure only several constituents of a chemically complex odor plume that includes, potentially, hundreds of volatile organic compounds.23
A further design limitation was the contemporaneous assessment of both exposure and outcome for the analyses of odor as a predictor of stress and negative mood. Because both exposure and outcome were assessed by self-report, it is difficult to determine how the assessment of one affected the assessment of the other. Participants spent 10 minutes outdoors before returning indoors to complete the required data collection activities; they rated the intensity of any malodor present and then rated stress and mood. Rating the odor while stressed or annoyed for reasons unrelated to odor may have induced a higher rating than the participant would have rated in the absence of feeling stressed or annoyed. Though the results of the analyses of odor and stress or mood must be interpreted in light of this design limitation, odor as a marker of exposure is important because it captures information on numerous other pollutants with odorant properties that we were unable to explicitly measure in this study. Furthermore, it permits consideration of the mixture of chemicals emitted from industrial hog operations as opposed to its individual constituent parts.
Conclusions
In a community-based, longitudinal study of neighbors of industrial hog operations, we observed associations among malodor, several airborne emissions, stress, and negative mood. Specifically, we observed increased reporting of stress and negative mood in response to increasing malodor. Additionally, increases in H2S and semivolatile PM10, both odorous in nature, were associated with reported stress and 1 or more mood variables. Our findings complement a large literature on malodor as an environmental stressor. Malodor and concomitant airborne emissions do appear to trigger stress and negative mood in nearby residents unwillingly exposed at home.
It is important to contextualize the effect of malodor on the lives of nearby residents. People who cannot afford air conditioning, clothes dryers, membership at a gym, and entertaining in restaurants depend on opening their windows for ventilation, drying their clothes outside, exercising in their yards, and entertaining family and friends in and around their homes. In ethnographic interviews, neighbors of industrial hog operations report that they refrain from gardening, walking, chores, and having cook-outs with family and friends because of hog odor, and they report interruption of their sleep because of hog odor inside their homes.54 This is significant, because physical activity, social support, and sleep are important for health. Industrial hog operations in North Carolina are located disproportionately in low income, African American communities35 that have limited financial resources to prevent the influx of polluting industries as well as to manage the impacts of uncontrollable environmental malodors on physical and mental health. Recognizing that health is a state of well-being, and not merely the absence of disease,40 public health and environmental professionals should consider the impacts of environmental malodor and its potential role in magnifying health disparities.
Acknowledgments
This work was supported by the Environmental Justice and Community-Based Participatory Research Program of the National Institute of Environmental Health Sciences (R01 ES011359) and by the Biostatistics for Research in Environmental Health Training Grant of the National Institute of Environmental Health Sciences (5-T32-ES07018).
We wish to thank the community-based organizations, whose names remain anonymous to protect the identity of the communities in which they worked, for their efforts in participant recruitment and retention throughout the study. We also thank the study participants who gave their time and energy to the project. We further acknowledge Steve Hutton, the project programmer, for his tireless work in preparing data sets for analysis.
Human Participant Protection
This study was approved annually by the institutional review board of the University of North Carolina at Chapel Hill. All study participants provided informed consent.
References
- 1.Asmus CL, Bell PA. Effects of environmental odor and coping style on negative affect, anger, arousal, and escape. J Appl Soc Psychol. 1999;29:245–260 [Google Scholar]
- 2.Avery RC, Wing S, Marshall SW, Schiffman SS. Odor from industrial hog farming operations and mucosal immune function in neighbors. Arch Environ Health. 2004;59(2):101–108 [DOI] [PubMed] [Google Scholar]
- 3.Both R, Sucker K, Winneke G, Koch E. Odour intensity and hedonic tone - important parameters to describe odour annoyance to residents? Water Sci Technol. 2004;50(4):83–92 [PubMed] [Google Scholar]
- 4.Bullers S. Environmental stressors, perceived control, and health: the case of residents near large-scale hog farms in Eastern North Carolina. Hum Ecol. 2005;33(1):1–16 [Google Scholar]
- 5.Carlsson F, Karlson B, Orbaek P, Osterberg K, Ostergren P. Prevalance of annoyance attributed to electrical equipment and smells in a Swedish population, and relationship with subjective health and daily functioning. Public Health. 2005;119:568–577 [DOI] [PubMed] [Google Scholar]
- 6.Cole D, Todd L, Wing S. Concentrated swine feeding operations and public health: a review of occupational and community health effects. Environ Health Perspect. 2000;108:685–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donham KJ, Wing S, Osterberg D, et al. Community health and socioeconomic issues surrounding concentrated animal feeding operations. Environ Health Perspect. 2007;115:317–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott SJ, Taylor SM, Walter S, Stieb D, Frank J, Eyles J. Modelling psychosocial effects of exposure to solid waste facilities. Soc Sci Med. 1993;37:791–804 [DOI] [PubMed] [Google Scholar]
- 9.Eyles J, Taylor S, Johnson N, Baxter J. Worrying about waste: living close to solid waste disposal facilities in Southern Ontario. Soc Sci Med. 1993;37:805–812 [DOI] [PubMed] [Google Scholar]
- 10.Heederik D, Sigsgaard T, Thorne P, et al. Health effects of airborne exposures from concentrated animal feeding operations. Environ Health Perspect. 2007;115:298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller K, Ball R. A Retrospective Study of Diarrheal and Respiratory Illness Incidence Rates in Milford, Utah, 1992-1998. Salt Lake City, Utah: Utah Department of Health, Bureau of Epidemiology; 2000 [Google Scholar]
- 12.Luginaah IN, Taylor SM, Elliott SJ, Eyles JD. A longitudinal study of the health impacts of a petroleum refinery. Soc Sci Med. 2000;50:1155–1166 [DOI] [PubMed] [Google Scholar]
- 13.Luginaah IN, Taylor SM, Elliott SJ, Eyles JD. Community reappraisal of the perceived health effects of a petroleum refinery. Soc Sci Med. 2002;55:47–61 [DOI] [PubMed] [Google Scholar]
- 14.Luginaah IN, Taylor SM, Elliott SJ, Eyles JD. Community responses and coping strategies in the vicinity of a petroleum refinery in Oakville, Ontario. Health Place. 2002;8:177–190 [DOI] [PubMed] [Google Scholar]
- 15.Merchant JA, Naleway AL, Svendsen ER, et al. Asthma and farm exposures in a cohort of rural Iowa children. Environ Health Perspect. 2005;113:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirabelli MC, Wing S, Marshall SW, Wilcosky TC. Race, poverty, and potential exposure of middle-school students to air emissions from confined swine feeding operations. Environ Health Perspect. 2006;114:591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirabelli MC, Wing S, Marshall SW, Wilcosky TC. Asthma symptoms among adolescents who attend public schools that are located near confined swine feeding operations. Pediatrics. 2006;118:e66–e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitloehner FM, Schenker MB. Environmental exposure and health effects from concentrated animal feeding operations. Epidemiology. 2007;18:309–311 [DOI] [PubMed] [Google Scholar]
- 19.Neutra R, Lipscomb J, Satin K, Shusterman D. Hypotheses to explain the higher symptom rates observed around hazardous waste facilities. Environ Health Perspect. 1991;94:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimmermark S. Odour influence on well-being and health with specific focus on animal production emissions. Ann Agric Environ Med. 2004;11:163–173 [PubMed] [Google Scholar]
- 21.Radon K, Peters A, Praml G, et al. Livestock odours and quality of life of neighbouring residents. Ann Agric Environ Med. 2004;11:59–62 [PubMed] [Google Scholar]
- 22.Radon K, Schulze A, Ehrenstein V, van Strein R, Praml G, Nowak D. Environmental exposure to confined animal feeding operations and respiratory health of neighboring residents. Epidemiology. 2007;18:300–308 [DOI] [PubMed] [Google Scholar]
- 23.Schiffman SS, Bennett JL, Raymer JH. Quantification of odors and odorants from swine operations in North Carolina. Agric Forest Meteorol. 2001;108:213–240 [Google Scholar]
- 24.Schiffman SS, Walker JM, Dalton P, et al. Potential health effects of odor from animal operations, wastewater treatment, and recycling of byproducts. J Agromed. 2000;7(1):7–81 [PubMed] [Google Scholar]
- 25.Schiffman SS. Livestock odors: implications for human health and well-being. J Anim Sci. 1998;76:1343–1355 [DOI] [PubMed] [Google Scholar]
- 26.Schiffman SS, Sattely Miller EA, Suggs MS, Graham BG. The effect of environmental odors emanating from commercial swine operations on the mood of nearby residents. Brain Res Bull. 1995;37:369–375 [DOI] [PubMed] [Google Scholar]
- 27.Shusterman D. Critical review: the health significance of environmental odor pollution. Arch Environ Health. 1992;47:76–87 [DOI] [PubMed] [Google Scholar]
- 28.Shusterman D, Lipscomb J, Neutra R, Satin K. Symptom prevalence and odor-worry interaction near hazardous waste sites. Environ Health Perspect. 1991;94:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinheider B, Winneke G. Industrial odours as environmental stressors: exposure annoyance associations and their modification by coping, age, and perceived health. J Environ Psychol. 1993;13:353–363 [Google Scholar]
- 30.Sucker K, Both R, Winneke G. Adverse effects of environmental odours: reviewing studies on annoyance responses and symptom reporting. Water Sci Technol. 2001;44(9):43–51 [PubMed] [Google Scholar]
- 31.Taylor SM, Elliott S, Eyles J, et al. Psychosocial impacts in populations exposed to solid waste facilities. Soc Sci Med. 1991;33:441–447 [DOI] [PubMed] [Google Scholar]
- 32.Thu K. Odor problems from large-scale agriculture: nuisance or public health problem? Health Environ Dig. 1998;12(8):57–60 [Google Scholar]
- 33.Thu KM. Public health concerns for neighbors of large-scale swine production operations. J Agric Saf Health. 2002;8(2):175–184 [DOI] [PubMed] [Google Scholar]
- 34.Thu K, Donham K, Ziegenhorn R, et al. A control study of the physical and mental health of residents living near a large-scale swine operation. J Agric Saf Health. 1997;3(1):13–26 [Google Scholar]
- 35.Wing S, Cole D, Grant G. Environmental injustice in North Carolina's hog industry. Environ Health Perspect. 2000;108:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wing S, Wolf S. Intensive livestock operations, health, and quality of life among Eastern North Carolina residents. Environ Health Perspect. 2000;108:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winneke G, Neuf M, Steinheider B. Separating the impact of exposure and personality in annoyance response to environmental stressors, particularly odors. Environ Int. 1996;22(1):73–81 [Google Scholar]
- 38.National Research Council Toward Environmental Justice: Research, Education, and Health Policy Needs. Washington, DC: National Academy Press; 1999 [PubMed] [Google Scholar]
- 39.Bullard RD, Wright BH. Environmental justice for all: community perspectives on health and research needs. Toxicol Ind Health. 1993;9:821–841 [DOI] [PubMed] [Google Scholar]
- 40. Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference, New York, 19–22 June, 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948. Geneva, Switzerland: World Health Organization; 1948. [Google Scholar]
- 41.Wing S, Horton R, Muhammad N, Grant G, Tajik M, Thu K. Integrating epidemiology, education, and organizing for environmental justice: community health effects of industrial hog operations. Am J Public Health. 2008;98:1390–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbie-Smith G, Thomas S, Williams M, Moody-Ayers M. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iowa State University and the University of Iowa Study Group Iowa Concentrated Animal Feeding Operations Air Quality Study. Iowa City, IA: University of Iowa; 2002. Available at: http://www.ehsrc.uiowa.edu/cafo_air_quality_study.html. Accessed November 6, 2008 [Google Scholar]
- 44.Wing S, Horton R, Marshall S, et al. Air pollution and odor in communities near industrial swine operations. Environ Health Perspect. 2008;116:1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James SA, Hartnett SA, Kalsbeek WD. John Henryism and blood pressure differences among black men. J Behav Med. 1983;6:259–278 [DOI] [PubMed] [Google Scholar]
- 46.James SA. John Henryism and the health of African-Americans. Cult Med Psychiatry. 1994;18:163–182 [DOI] [PubMed] [Google Scholar]
- 47.E 544-75: Annual Book of ASTM Standards. West Conshohocken, PA: American Society for Testing and Materials; 1997 [Google Scholar]
- 48.Cohen S, Kessler R, Gordon L. Strategies for measuring stress in studies of psychiatric and physical disorders. : Cohen S, Kessler R, Gordon L, Measuring Stress: A Guide for Health and Social Scientists. New York, NY: Oxford University Press; 1997 [Google Scholar]
- 49.Littman AJ, White E, Satia JA, Bowen DJ, Kristal AR. Reliability and validity of 2 single-item measures of psychosocial stress. Epidemiology. 2006;17:398–403 [DOI] [PubMed] [Google Scholar]
- 50.McNair D, Loor M, Droppleman L. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971 [Google Scholar]
- 51.Seeber A, van Threil C, Haumann K, Kiesswetter E, Blaszkewicz M, Golka K. Psychological reactions related to chemosensory irritation. Int Arch Occup Environ Health. 2002;75:314–325 [DOI] [PubMed] [Google Scholar]
- 52.Smeets MA, Mauté C, Dalton PH. Acute sensory irritation from exposure to isopropanol (2-propanol) at TLV in workers and controls: objective versus subjective effects. Ann Occup Hyg. 2002;46:359–373 [DOI] [PubMed] [Google Scholar]
- 53.Smeets M, Dalton P. Perceived odor and irritation of isopropanol: a comparison between naive controls and occupationally exposed workers. Int Arch Occup Environ Health. 2002;75:541–548 [DOI] [PubMed] [Google Scholar]
- 54.Tajik M, Muhammad N, Lowman A, Thu K, Wing S, Grant G. Impact of odor from industrial hog operations on daily living activities. New Solut. 2008;81:193–205 [DOI] [PubMed] [Google Scholar]


