Abstract
Nicotinamide adenine dinucleotide (NAD+) extends longevity in experimental organisms, raising interest in its impact on human health. De novo NAD+ biosynthesis from tryptophan is evolutionarily conserved yet considered supplanted among higher species by biosynthesis from nicotinamide (Nam). Here we show that a bottleneck enzyme in de novo biosynthesis, quinolinate phosphoribosyltransferase (QPRT), defends renal NAD+ and mediates resistance to acute kidney injury (AKI). Following murine AKI, renal NAD+ fell, quinolinate rose, and QPRT declined. QPRT+/− mice exhibited higher quinolinate, lower NAD+, and higher AKI susceptibility. Metabolomics proposed elevated urinary quinolinate/tryptophan (uQ:T) as an indicator of reduced QPRT. Elevated uQ:T predicted AKI and other adverse outcomes in critically ill patients. A Phase 1 placebo-controlled study of oral Nam demonstrated dose-related increase in circulating NAD+ metabolites. Nam was well-tolerated and was associated with less AKI. Impaired NAD+ biosynthesis may therefore be a feature of high-risk hospitalizations for which NAD+ augmentation could be beneficial.
Keywords: acute kidney injury, NAD+, quinolinate, QPRT, nicotinamide, clinical trial
INTRODUCTION
NAD+ is a universal electron acceptor from glycolysis and the Krebs cycle. It is also a substrate for non-redox enzymes that consume NAD+ such as polyADP ribose polymerases (PARPs), sirtuins, and ectonucleotidases.1–5 Chronic deficiency of Nam or nicotinic acid, vitamin B3 analogs that are NAD+ precursors, affects several metabolically active organs. Deficiency of a third NAD+ precursor, tryptophan, can develop from inherited defects in a neutral amino acid transporter and also impairs metabolically active organs. Although the biosynthesis of NAD+ proceeds through distinct routes depending upon the dietary precursor, evidence from patients with dietary Nam deficiency or tryptophan transporter defects demonstrates that supplementation with one precursor can effectively treat deficiency of the other.5,6 More recently, NAD+ metabolism (Fig 1a) has been suggested as a therapeutic target for diverse diseases ranging from diet-induced obesity to neuronal degeneration and glaucoma, conditions that share substantial metabolic stress.7–9 NAD+ preservation may also prevent age-related declines in health and even extend lifespan.10–15 Critically ill patients are often subjected to severe metabolic stress—arising from systemic inflammation and ischemia—and are susceptible to an array of age-associated complications. One such complication, acute kidney injury (AKI), affects 3–10% of all hospitalized adults, can be deadly, and lacks a specific treatment.16
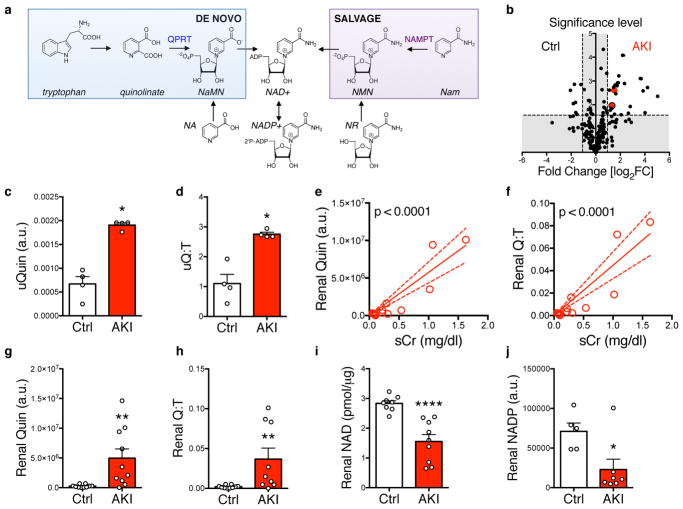
Figure 1. Renal and urinary quinolinate elevation in ischemic AKI.
(a) NAD+ biosynthetic pathways: (1) “de novo” from tryptophan (Trp) through the intermediate quinolinate (Quin); (2) via a “salvage” pathway from nicotinamide (Nam); (3) from nicotinic acid (NA); and (4) from nicotinamide riboside (NR). QPRT = quinolinate phosphoribosyltransferase. NAMPT = Nam phosphoribosyltransferase. NaMN = nicotinate mononucleotide, NMN = nicotinamide mononucleotide. (b) Volcano plot comparing urinary metabolites (n = 204) in mice 24h after sham operation or transient bilateral renal ischemia (AKI) (n = 4 animals/group). Vertical dashed lines indicate threshold for two-fold abundance difference. Horizontal dashed line indicates p = 0.05 threshold. X axis = log2[fold change for right condition/left condition]. Red dot = quinolinate, red dot with black border = quinolinate/tryptophan ratio. Y axis = -log10[p-value]. P-value computed by two-sided unpaired t-test without adjustment for multiple comparisons. (c,d) Urinary quinolinate (uQuin, a.u. = arbitrary unit) and urinary quinolinate/tryptophan (uQ:T) ratio from (b). (e,f) Renal tissue quinolinate and quinolinate/tryptophan (Q:T) ratio as a function of serum creatinine (sCr) determined 24h after no operation, sham operation or varying durations of transient renal ischemia (n = 13 animals). Correlations by Pearson method. (g,h) Renal tissue quinolinate content and renal quinolinate/tryptophan ratio in controls or mice 24h after 25 minutes transient renal ischemia (n = 10/group). (i,j) Renal tissue NAD+ (n = 8,9 respectively) and NADP+ content (n = 5,7 respectively) in controls or mice 24h after transient renal ischemia. Data in c,d,g–j displayed as mean ± SEM; pairwise comparisons by Mann-Whitney with two-sided *p < 0.05, **p < 0.01, ****p < 0.0001.
RESULTS
Renal and urinary quinolinate elevation in ischemic AKI
To study AKI, we conducted an unbiased metabolomics screen on the urine of mice with AKI induced by transient renal ischemia (Fig 1b). Of 204 metabolites measured (Supplemental Table 1), 27 were more than two-fold increased in post-ischemic urines compared to controls including several sugars and amino acids, a pattern consistent with tubular impairment (Supplemental Table 2). Among these metabolites was quinolinate, an intermediate in the de novo NAD+ biosynthetic pathway from tryptophan (Fig 1c). After normalizing for tryptophan, urinary quinolinate elevation persisted (Fig 1d). To assess whether excess urinary quinolinate reflected intrarenal processes rather than filtration into urine from extrarenal sources, we then measured kidney tissue levels. Both renal quinolinate and the ratio of renal quinolinate to renal tryptophan were strongly related to renal function and post-ischemic injury (Fig 1e–h). This suggested a reduction in AKI of renal QPRT, an enzyme that connects the initial steps of tryptophan’s pyrrole ring opening to the final steps of NAD+ biosynthesis (Fig 1a). Whereas quinolinate can only be transformed to penultimate NAD+ precursors, tryptophan and other upstream metabolites have multiple metabolic fates, thus making quinolinate the first fully committed NAD+ precursor in de novo biosynthesis (genome.jp/kegg). Renal NAD+ and renal NADP+ were also significantly reduced by AKI (Fig 1i,j).
QPRT mediates resistance to AKI
Data from public repositories demonstrated selective enrichment of human and mouse QPRT in the kidney and liver among the body’s major organs (Supplemental Fig 1a,b). Within the kidney, QPRT protein was abundant in the epithelium of the proximal tubule, its most metabolically active cellular compartment (Supplemental Fig 1c). Renal QPRT expression was attenuated by transient renal ischemia (Fig 2a), attenuated in mice hypersensitive to renal ischemia, and induced in mice resistant to renal ischemia (Supplemental Fig 1d).17
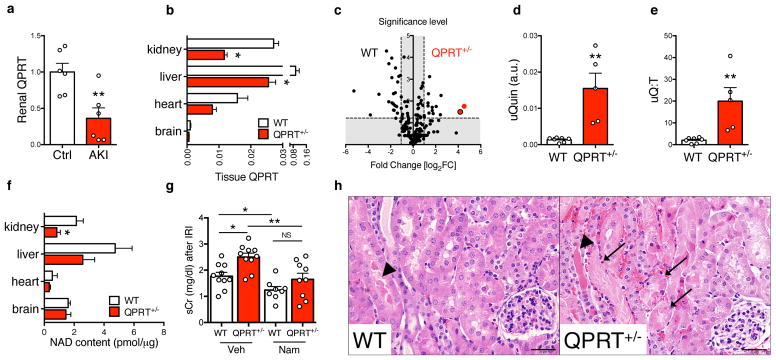
Figure 2. QPRT mediates resistance to experimental AKI.
(a) Mouse renal QPRT mRNA 24h after transient ischemia (n = 6 animals/group). (b) QPRT mRNA in littermate controls (WT) vs. QPRT+/− mice (n =5 animals/group). (c) Volcano plot comparing urinary metabolites (n = 204) in littermate controls (WT = 4 animals) vs. QPRT+/− mice (n = 5 animals). Vertical dashed lines indicate threshold for two-fold abundance difference. Horizontal dashed line indicates p = 0.05 threshold. X axis = log2[fold change for right condition/left condition]. Red dot = quinolinate, red dot with black border = quinolinate/tryptophan ratio. Y axis = -log10[p-value]. P-value computed by two-sided unpaired t-test without adjustment for multiple comparisons. (d,e) Urinary quinolinate (uQuin, a.u. = arbitrary unit) and urinary quinolinate/tryptophan (uQ:T) ratio from (c). (f) Tissue NAD+ content in littermate controls (WT) vs. QPRT+/− mice (n =5 animals/group). (g) Renal function 24h after transient renal ischemia in littermate controls (WT) vs. QPRT+/− mice receiving vehicle or Nam (400 mg/kg ip) −24h, −1h, and +4–6h relative to surgery (n = 10, 10, 8, and 9 animals/group respectively). (h) Representative examples from 3 independent animals per group of intratubular cast (arrowhead) and tubular necrosis (arrows) 24h after transient renal ischemia in littermate controls (WT) vs. QPRT+/− mice. Scale bar = 20 μm. Data in a,b,d–f,g displayed as mean ± SEM; pairwise comparisons by Mann-Whitney with two-sided *p < 0.05, **p < 0.01.
Involvement of QPRT in AKI has not been previously described. We therefore created QPRT+/− mice by CRISPR/Cas9 gene editing and studied several founder lines (Supplemental Fig 2). Loss of one allele recapitulated the extent of QPRT reduction associated with AKI (Fig 2b). Urinary quinolinate and urinary quinolinate/tryptophan ratio (uQ:T) were elevated in QPRT+/− mice even in the absence of renal injury. This corroborated the specificity of the AKI metabolite profile for reduced QPRT that was originally suggested by the biochemistry, namely that quinolinate is only known to be made and utilized in this single pathway of de novo NAD+ biosynthesis (Fig 1c–e and Supplemental Table 3). QPRT reduction lowered NAD+ in the kidney among several metabolically active organs (Fig 2f). This result suggested that the kidney utilizes de novo NAD+ biosynthesis for maintenance of normal NAD+ content. Based upon the renal NAD+ deficiency, we hypothesized that QPRT+/− mice would be more vulnerable to acute ischemic stress. Renal function and injury to tubular cells (Fig 2g,h) 24 hours after transient renal ischemia was indeed worse in QPRT+/− mice. We then tested the role of QPRT-independent augmentation of NAD+ metabolism via salvage biosynthesis by administering Nam in this model.17 Nam overcame the sensitivity of QPRT+/− mice to transient renal ischemia (Fig 2g). Together, these data showed that impairment of the de novo NAD+ biosynthetic pathway at QPRT not only characterizes AKI, but that this single enzymatic change lowers NAD+ in the kidney and increases susceptibility to local ischemic stress in an NAD+-dependent fashion.
uQ:T elevation in human AKI
In both mice and humans, quinolinate is only generated by spontaneous non-enzymatic cyclization of its immediate precursor in de novo NAD+ biosynthesis, 2-amino-β-carboxymuconate-ε-semialdehyde (2-ACMS), and quinolinate is only utilized as a substrate by QPRT (Fig 3a). The metabolic pathways therefore suggest that elevated quinolinate should be a highly specific indicator for the effect of reduced QPRT on flux through this pathway, and the results of Figs 1,2 support this biochemical prediction. In a prospectively enrolled cohort of patients exposed to renal ischemia by on-pump cardiac surgery (Supplemental Table 4), we therefore performed a nested case-control study to assess relationships between metabolites of the de novo pathway and AKI (Supplemental Table 5 and Supplemental Fig 3). Measurement of sequential metabolites of the de novo pathway in the urine revealed a transition to higher urinary quinolinate levels among those individuals who subsequently developed AKI compared to those free of post-operative AKI (Fig 3b–e and Supplemental Fig 4). Similarly, elevated uQ:T developed shortly after the operation and persisted through post-operative day 5 (Fig 3f). These results suggested that the ischemic human kidney shares de novo NAD+ biosynthetic impairment with its murine counterpart and that this can be non-invasively monitored by uQ:T.
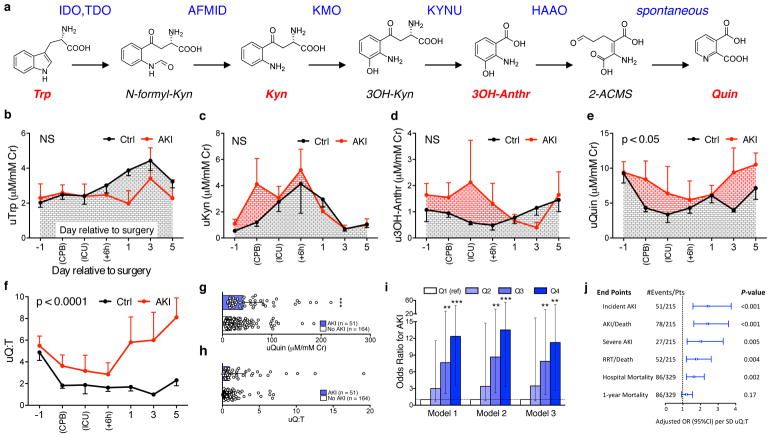
Figure 3. Urinary quinolinate/tryptophan (uQ:T) elevation in human AKI.
(a) De novo biosynthesis of NAD+ from tryptophan. IDO=indole dioxygenase; TDO-tryptophan dioxygenase; AFMID = arylformamidase; KMO = kynurenine monoxygenase; KYNU = kynureninase; HAAO = hydroxyanthranilate dioxygenase; Quinolinate is then ribosylated by QPRT = quinolinate phosphoribosyltransferase. (b–f) Urinary metabolites in cardiac surgery patients. Measurements were compared by time relative to cardiopulmonary bypass and whether subjects developed AKI (n=6/group). CPB = during cardiopulmonary bypass. ICU = immediate post-operative period during which patients were still intubated for mechanical ventilation. +6h = 6 hours since surgery completion. Otherwise, times are day relative to surgery. For b–f, significance was assessed by two-way ANOVA with two-sided p-value indicating treatment effect and data displayed as mean ± SEM. (g–i) Urinary metabolites in a prospective cohort study of ICU patients (n = 215 subjects, 51 of whom subsequently developed AKI and 164 without AKI). (g,h) Urinary quinolinate and uQ:T in those who did or did not develop AKI. Data displayed as median ± interquartile range and compared by Wilcoxon Rank Sum test. (i) Odds ratios for incident AKI (n = 215 subjects) according to quartiles of uQ:T determined by multivariate logistic regression. Model 1 is unadjusted. Model 2 is adjusted for the following demographics and comorbidities: age, gender, race, baseline estimated glomerular filtration rate, hypertension, and diabetes mellitus. Model 3 is further adjusted for the following severity of illness covariates: ICU type, need for mechanical ventilation, and APACHE II score. Quartile 1 (Q1) was the reference in all models. Error bars for 95% confidence interval. (j) Forest plot for incident AKI and other outcomes as listed per standard deviation (SD) of log-transformed uQ:T. Error bars for 95% confidence interval. Two-sided **p < 0.01, ***p < 0.001.
In low-income countries, AKI may affect 40–50% of all hospitalized individuals; even in high-income countries, AKI affects 30% of intensive care unit (ICU) patients.18 Compared to the more homogeneous stressor of cardiac surgery, AKI in the ICU often arises from ischemia compounded by toxic drugs and severe infections. Thus, to assess the generalizability of our findings from the cardiac surgery cohort, we next studied uQ:T in a heterogeneous ICU population (Supplemental Table 6).19 Among 215 subjects from a prospectively collected cohort of 329 ICU subjects (Supplemental Fig 5) who had urine samples available by a median of 2.5 days prior to the development of AKI, urinary quinolinate and uQ:T were higher than those who did not develop AKI (Fig 3g,h). Further, serial quartiles of uQ:T were associated in a monotonic fashion with increased risk of incident AKI in univariate analyses and in multivariable models adjusted for major confounders including baseline renal function (Fig 3i). The most stringent of these models (Model 3) adjusted for age, gender, race, baseline kidney function, hypertension, diabetes, and severity of illness as measured by type of ICU, need for mechanical ventilation, and a severity of illness score termed APACHE (Acute Physiology and Chronic Health Evaluation). Even in this model, higher uQ:T levels were was associated with significantly increased probability of a spectrum of adverse outcomes, including hospital mortality (Fig 3j and Supplemental Table 7). These results suggested that humans at risk for AKI develop a block at QPRT just as mice do, and that a non-invasive metabolic signature of reduced QPRT may help identify this biological disturbance in clinically relevant settings.
QPRT-independent NAD+ precursor treatment
If transient NAD+ biosynthetic deficiency is a risk factor for AKI, then NAD+ augmentation could be beneficial. Among several NAD+ precursors, we chose to test Nam because (A) Nam’s ability to increase NAD+ is independent of QPRT (Fig 1a); (B) systemic Nam increases renal NAD+;17 (C) Nam administration overcame the sensitivity of QPRT+/− mice to renal ischemia (Fig 2h); and (D) Nam is reported to be well-tolerated at high doses administered chronically.20–22 We first tested a three-day regimen of 3 gm/day of oral Nam among healthy volunteers in order to confirm that the pharmaceutical supply of Nam led to measurable changes in circulating Nam (Supplemental Table 8). We were able to detect elevation of circulating Nam, consistent with a previous report of oral Nam obtained from different manufacturers (Supplemental Fig 6a).23 Serum creatinine did not change among recipients, ruling out potential interference of high circulating Nam levels with the creatinine determination methodology (Supplemental Fig 6b).
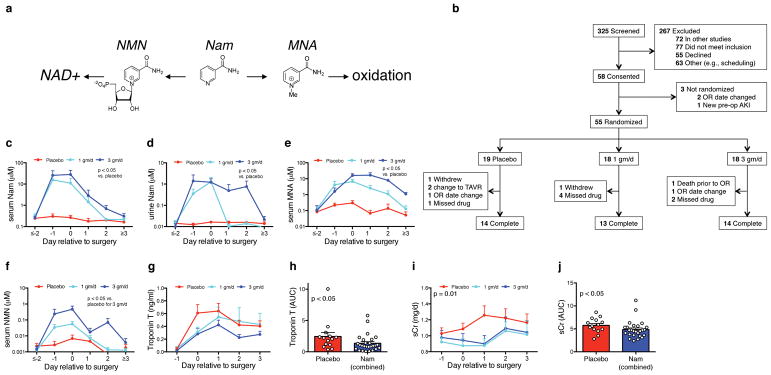
We then designed a Phase I pilot study of oral Nam administration among adults undergoing cardiac surgery (Clinicaltrials.gov identifier NCT02701127, Fig 4a,b). The primary endpoint compared blood Nam levels between blinded participants randomized to receive placebo, 1 gm/day Nam, or 3 gm/day Nam once daily by mouth or orogastric tube on days −1, 0, and +1 relative to surgery. The treatment groups were well-balanced across 37 baseline demographic, clinical, and laboratory features (Table 1 and Supplemental Tables 9,10). Nam administration significantly increased blood and urine Nam (Fig 4c,d). Nam can either contribute to de novo NAD+ biosynthesis through the intermediate Nam mononucleotide (NMN) or undergo methylation to N1-methyl-Nam (MNA) followed by irreversible oxidation to waste products. Nam administration at the 3 gm/day regimen increased NMN whereas either Nam regimen increased MNA relative to placebo (Fig 4e,f). Nam administration was not associated with increased adverse events compared to placebo. Serious adverse events were uncommon, were distributed evenly across the study arms, and were independently adjudicated to be unrelated to study participation (Table 2).
Figure 4. Participants and outcomes of oral Nam Phase 1 pilot study in cardiac surgery patients.
(a) Nam can be converted to NAD+ through the intermediate nicotinamide mononucleotide (NMN) or methylated and oxidized for excretion through the intermediate N1-methyl nicotinamide (MNA). (b) Flow diagram for participants in oral Nam Phase 1 pilot study (clinicaltrials.gov entry NCT02701127). (c–f) Serum Nam, urine Nam, serum MNA, and serum NMN temporal profiles by treatment arm with data displayed as mean ± SEM. N per group as indicated in (b). P-values for c–f are pairwise Mann-Whitney comparisons of areas under the curve (AUCs, μM * days) for each treatment arm relative to placebo. For f, only the 3 gm/d arm was significantly different vs. placebo. (g,h) Temporal profiles of the cardiac injury marker Troponin T by treatment arm displayed as mean ± SEM and AUCs (ng/ml * days) with treatment groups combined (n = 14 in placebo, n = 27 in Nam treatment groups combined). (i,j) Serum creatinine (sCr) by treatment arm displayed as mean ± SEM and AUCs (mg/dl * days) with treatment groups combined (n = 14 in placebo, n = 27 in Nam treatment groups combined). AUCs for troponin T and sCr were compared to placebo by Mann-Whitney.
Table 1. Baseline characteristics for oral Nam Phase 1 pilot study in cardiac surgery patients.
Baseline demographic and clinical parameters (clinicaltrials.gov entry NCT02701127). There were no statistically significant between-group differences in any of the listed variables. BMI=body mass index, CKD = chronic kidney disease, CABG = coronary artery bypass graft, BUN = blood urea nitrogen, eGFR = estimated glomerular filtration rate, WBC = white blood cell count, AST = aspartate aminotransferase, ALT = alanine aminotransferase, LDH = lactate dehydrogenase, CRP = C-reactive protein, CK-MB = creatine kinase-muscle brain fraction, CK = creatine kinase, PT = prothrombin time, PTT = partial thromboplastin time, INR = international normalized ratio.
| Baseline Characteristics | Treatment Group | Placebo Group (n=14) | ||
|---|---|---|---|---|
|
| ||||
| Nam 1 g/d (n=13) | Nam 3 g/d (n=14) | Combined (n=27) | ||
|
|
||||
| Patient Characteristics | ||||
| Age, y, median (IQR) | 52 (48.5–70.5) | 64.5 (56–72.5) | 58 (52–72) | 58 (53.5–67.5) |
| BMI, kg/m2, mean (SD) | 31 (8) | 29 (5.3) | 30 (6.7) | 34 (6.8) |
| Female gender, n (%) | 4 (31) | 1(7) | 5 (19) | 1(7) |
| African American, n (%) | 1 (8) | 1(7) | 2 (7) | 0 |
| CKD, eGFR≤45 ml/min or proteinuria, n (%) | 1 (8) | 3 (21) | 4 (15) | 2 (14) |
| Cleveland Clinic Score ≥5, n (%) | 3 (23) | 0 | 3 | 1 (7) |
| Ejection Fraction <35%, n (%) | 1 (8) | 1(7) | 2 (7) | 1 (7) |
| Hypertension, n (%) | 8 (62) | 10(71) | 18 (67) | 12 (86) |
| Heart failure, n (%) | 4 (31) | 4(29) | 8 (30) | 4 (29) |
| Diabetes, n (%) | 5 (38) | 2(14) | 7 (26) | 6 (43) |
| Tobacco use, n (%) | 9 (69) | 8(57) | 17 (63) | 9 (64) |
| Surgical Characteristics | ||||
| Aortic cross-clamp time, min., mean (SD) | 60±27 | 78±37 | 69±33 | 73±21 |
| CABG, n (%) | 6 (46) | 5 (36) | 11 (41) | 7 (50) |
| CABG+Valve, n (%) | 3 (23) | 1 (7) | 4 (15) | 1 (7) |
| Valve, n (%) | 4 (31) | 8 (57) | 12 (44) | 6 (43) |
| Previous Heart Surgery, n (%) | 0 | 2 (14) | 2 (7) | 2 (14) |
| Laboratory Characteristics | ||||
| Sodium, mEq/L, mean (SD) | 137±2 | 138±3 | 138±2 | 139±3 |
| Potassium, mEq/L, mean (SD) | 4.1±0.4 | 4±0.4 | 4.0±0.4 | 4.2±0.4 |
| Bicarbonate, mEq/L, mean (SD) | 25±3 | 25±2 | 25±3 | 26±2 |
| BUN, mg/dL, mean (SD) | 22±5 | 17±4 | 19±6 | 17±5 |
| Creatinine, mg/dL, median (IQR) | 1 (0.7–1.1) | 0.9 (0.7–1.2) | 0.9 (0.7–1.1) | 1.1 (0.8–1.2) |
| eGFR, ml/min, median (IQR) | 83 (69–103) | 86 (67–98) | 85 (68–100) | 76 (62–103) |
| Hematocrit, %, mean (SD) | 40±5 | 40±4 | 40±5 | 39±3 |
| Hemoglobin, g/dL, mean (SD) | 13±1.9 | 13.3±1.7 | 13.2±1.8 | 12.8±1.3 |
| Platelets, k/μL, mean (SD) | 208±42 | 188±67 | 198±56 | 202±56 |
| WBC, k/μL, mean (SD) | 7.3±1.7 | 7.6±2.5 | 7.5±2.1 | 7.0±1.9 |
| AST, IU/L, mean (SD) | 27±18 | 31±28 | 29±23 | 28±11 |
| ALT, IU/L, mean (SD) | 28±21 | 29±22 | 29±21 | 30±19 |
| Total Bilirubin, mg/dL, mean (SD) | 0.5±0.2 | 0.7±0.2 | 0.6±0.3 | 0.6±0.3 |
| LDH, IU/L, mean (SD) | 249±86 | 244±105 | 246±95 | 228±51 |
| CRP, mg/L, mean (SD) | 18±25 | 12±25 | 15±25 | 8±13 |
| Troponin T, ng/mL, median (IQR) | 0.02 (0.01–0.03) | 0.01 (0.01–0.01) | 0.01 (0.01–0.03) | 0.01 (0.01–0.17) |
| CK-MB, ng/mL, median (IQR) | 2 (2–3) | 2 (2–5) | 2 (2–5) | 2 (1–6) |
| CK, IU/L, median (IQR) | 140 (64–194) | 113 (52–210) | 131 (64–194) | 84 (73–126) |
| PT, sec., mean (SD) | 12±2 | 12±1 | 12±2 | 12±1 |
| PTT, sec., mean (SD) | 51±25 | 45±22 | 48±23 | 40±17 |
| INR, mean (SD) | 1.1±0.2 | 1.1±0.1 | 1.1±0.1 | 1.1±0.1 |
Table 2.
Perioperative assessments and adverse events for oral Nam Phase 1 pilot study in cardiac surgery patients.
| Safety Monitoring | Treatment Group | Placebo Group (n=14) | P-value† | ||
|---|---|---|---|---|---|
|
| |||||
| Nam 1 g/d (n=13) | Nam 3 g/d (n=14) | Combined (n=27) | |||
|
|
|||||
| Perioperative Assessment | |||||
| Intra-OP UOP, ml/kg/hr, median (IQR) | 5.4 (2.2–6.9) | 1.7 (0.5–5.1) | 3.6 (1.6–6.8) | 2.4 (1.4–4.3) | 0.581 |
| Intra-op total volume administration, ml, median (IQR) | 3520 (2988–3704) | 3475 (3088–4419) | 3520 (3100–4050) | 3690 (3330–4060) | 0.518 |
| Hospital stay, d, median (IQR) | 8 (7–10) | 9 (8–10) | 8 (7–10) | 10 (5–11) | 0.831 |
| Post OP hospital stay, d, median (IQR) | 5 (4–7) | 6 (4–8) | 5 (4–8) | 5 (4–7) | 0.720 |
| ICU stay, d, median (IQR) | 2 (1–5) | 2 (1–3) | 2 (1–4) | 2 (1–3) | 0.826 |
| ALT, IU/L, mean (SD) | |||||
| Day 0 | 31±26 | 29±14 | 30±20 | 28±20 | 0.793 |
| Day 1 | 23±18 | 20±10 | 21±14 | 23±17 | 0.747 |
| Day 2 | 17±11 | 17±7 | 17±9 | 18±12 | 0.684 |
| Day 3 | 14±7 | 22±19 | 18±15 | 19±9 | 0.814 |
| AST, IU/L, mean (SD) | |||||
| Day 0 | 32±21 | 40±15 | 36±18 | 35±13 | 0.848 |
| Day 1 | 42±23 | 46±20 | 44±21 | 35±13 | 0.208 |
| Day 2 | 37±19 | 40±22 | 38±20 | 37±23 | 0.838 |
| Day 3 | 27±12 | 35±20 | 32±17 | 33±20 | 0.877 |
| LDH, IU/L, mean (SD) | |||||
| Day 0 | 222±81 | 267±57 | 244±72 | 271±104 | 0.409 |
| Day 1 | 260±89 | 300±96 | 280±92 | 287±79 | 0.843 |
| Day 2 | 325±165 | 349±220 | 339±194 | 298±87 | 0.502 |
| Day 3 | 279±110 | 284±89 | 282±96 | 278±67 | 0.916 |
| CK-MB, ng/mL, median (IQR) | |||||
| Day 0 | 14 (4–17) | 16 (5–26) | 15 (4–19) | 20 (12–41) | 0.020 |
| Day 1 | 13 (9–28) | 16 (5–26) | 16 (11–28) | 18 (9–27) | 0.490 |
| Day 2 | 5 (3–11) | 5 (3–10) | 5 (3–10) | 7 (3–17) | 0.242 |
| Day 3 | 3 (1–6) | 3 (2–5) | 3 (1–5) | 2 (1–5) | 0.294 |
| CK, IU/L, median (IQR) | |||||
| Day 0 | 144 (132–215) | 186 (124–486) | 172 (132–417) | 247 (177–445) | 0.538 |
| Day 1 | 507 (266–740) | 577 (279–737) | 542 (276–738) | 414 (371–569) | 0.268 |
| Day 2 | 359 (119–959) | 484 (202–1614) | 427 (176–958) | 392 (331–588) | 0.354 |
| Day 3 | 328 (114–627) | 243 (92–535) | 279 (111–595) | 187 (131–198) | 0.218 |
| Adverse Events | |||||
| Nausea a, n (%) | 3 (23) | 2 (14) | 5 (19) | 3 (21) | 0.673 |
| Poor Appetite b, n (%) | 3 (23) | 2 (14) | 5 (19) | 4 (29) | 0.692 |
| Rash c, n (%) | 0 | 0 | 0 | 0 | |
| Delirium, n (%) | 1 (8) | 1 (7) | 2 (7) | 1 (7) | >0.999 |
| Heart Failure d, n (%) | 2 (15) | 1 (7) | 3 (11) | 1 (7) | >0.999 |
| Fever, n (%) | 1 (8) | 1 (7) | 2 (7) | 1 (7) | >0.999 |
| Serious Adverse Events | |||||
| 30 day Re-Hospitalization (unrelated to study participation) | 5 e, f, g, h, i | 0 | 5 | 1 j | - |
| Dialysis | 0 | 0 | 0 | 0 | - |
| Death | 0 | 0 | 0 | 0 | - |
| Complete Heart Block | 0 | 1 k | 1 | 0 | - |
| Hematothorax | 0 | 0 | 0 | 1 l | - |
P-Values were computed between combined treatment group and placebo group by Mann-Whitney (continuous variables) or chi-square test (categorical variables). In order to enhance detection of between-group differences, no adjustments were made for multiple comparisons
GI symptoms, including nausea were the most common adverse drug effects of NAM in past clinical trials, and therefore patients were specifically asked on each study visit through day 30.
Rash, a common symptom of niacin, and infrequently reported to occur with Nam was not reported or identified during study visits.
Heart failure beyond general post-operative fluid overload, requiring diuresis after discharge from intensive care unit occurred in all 3 treatment arms and was adjudicated as unrelated to study participation.
Left flank pain, likely musculoskeletal.
Sternal wound infection.
Diabetes and heart failure exacerbation, and diabetic foot infection.
Heart failure exacerbation.
Pulmonary embolus 2 weeks post-operation in setting of morbid obesity and prolonged immobilization.
Pneumonia.
Complete heart block on post-operative day (POD) 2, and resolved on POD 4.
POD 1 return to operating room for intrathoracic bleed. Full recovery and discharge by POD 9. Unrelated to study participation.
Given (A) that either Nam treatment arm yielded significant higher exposure to Nam than the placebo arm, (B) the comparable safety of either Nam dose to placebo, and (C) the comparable effect on serum creatinine of either Nam dose, we also combined the two Nam arms into one treatment group. One of the safety assessments evaluated whether Nam increased perioperative cardiac injury. Nam treatment was associated with lower blood levels of the cardiac injury marker troponin T as compared to placebo (Fig 4g,h). In a second safety assessment, we examined renal function since cardiac surgery increases AKI risk. Nam was associated with better estimated renal function compared to placebo (Fig 4i,j). AKI events were significantly lower with Nam treatment than placebo (Supplemental Table 11 and Supplemental Fig 7). Taken together, short-term Nam administration increased Nam levels and appeared to be well-tolerated among cardiac surgery patients. Safety assessments linked Nam administration to lower AKI risk.
DISCUSSION
The present studies investigated the metabolic changes associated with AKI by applying a localized injury model in mice and unbiased metabolomic screening. A highly specific indicator of reduced QPRT emerged. Mimicking this acquired defect by gene editing demonstrated that reduced QPRT lowers renal NAD+, raises urinary quinolinate, and exacerbates AKI susceptibility. These results established QPRT as a mediator of renal stress resistance. We then conducted four independent human studies to investigate quinolinate’s utility as a urinary indicator of diminished de novo NAD+ biosynthesis and the potential for QPRT-independent NAD+ augmentation: (1) urine metabolites from the de novo NAD+ biosynthetic pathway in a cardiac surgery discovery cohort (n = 12); (2) urine metabolites from an independent prospectively collected ICU cohort (n = 329); (3) oral Nam at the highest tested dose administered to healthy volunteers (n = 8); and (4) a Phase 1 pilot study of oral Nam in cardiac surgery patients (n = 41). The results propose that de novo NAD+ biosynthesis becomes impaired during human AKI and that augmentation of NAD+ metabolism may be safe and potentially beneficial.
These findings move the growing field of NAD+ metabolism research into a human disease context. Among metabolically active organs, kidney function peaks at approximately age 30, and then steadily declines for unclear reasons. When AKI develops in an elderly patient, the acute event may take place against a backdrop of diminished NAD+ biosynthetic reserve. Yet the majority of patients suffering transient metabolic stressors do not develop AKI. Those spared from AKI may have superior NAD+ metabolism from better nutrition, a diminished tendency toward stress-dependent suppression of QPRT, or both. Elevating NAD+ metabolism may have several beneficial mechanisms of action, such as post-ischemic augmentation of fatty acid oxidation—for which NAD+ levels are rate-limiting24—to the provision of substrate for cytoprotective sirtuin enzymes.25 NADP+ may also be critical in AKI as its reduced form, NADPH, promotes detoxification of reactive oxygen species.26 Parsing a singular kidney-protective mechanism downstream of NAD+ augmentation may be challenging given the ~400 redox reactions involving NAD+/NADH, ~50 reactions involving NAD+ consumption, and ~30 redox reactions involving NADP+/NADPH throughout the cell.27
The de novo NAD+ biosynthetic pathway has been considered a minor contributor to intracellular NAD+ levels in mammals. However, a recent study identified rare loss-of-function mutations in two other de novo enzymes, 3-hydroxyanthranilate-3,4-dixoygenase (HAAO) and kynureninase (KYNU) that reduced NAD+ in humans. Not only did this study independently corroborate the quantitative contribution of de novo biosynthesis to NAD+ metabolism, but affected individuals exhibited major renal anomalies.28 This report identified a pivotal role for de novo NAD+ biosynthesis during human development and proposed the therapeutic potential of “orthogonal” NAD+ augmentation during gestation, i.e., independent of the de novo pathway. In the present context of acquired de novo NAD+ impairment related to reduced QPRT, there is a striking parallel in that kidney involvement is one of the most common complications suffered by critically ill patients and may also respond to orthogonal NAD+ augmentation. The present data propose both a mechanism of locally diminished NAD+ biosynthesis triggered by metabolic stressors such as ischemia and an inexpensive strategy for its therapeutic replenishment. Further, acquired impairments in the de novo pathway associated with aging or chronic kidney disease—two major AKI risk factors—may reduce renal NAD+ and thereby enhance susceptibility to AKI.
The transcriptional co-activator PPAR-gamma-coactivator-1-alpha (PGC1α) coordinates expression of QPRT and other de novo enzymes in the kidney both at baseline (Supplemental Fig 1d) and during ischemic AKI, but other QPRT regulators may also be important. For example, enrichment of QPRT expression in the kidney and liver may relate to their shared blood detoxification function. Shortage of NAD+ in the kidney as a consequence of QPRT reduction could also be a deleterious side effect of an otherwise adaptive response to injury. Since reduced QPRT would favor the accumulation of earlier metabolites in the de novo pathway such as kynurenine, reduction of QPRT may offer the injured kidney a chemical mechanism to modulate local signaling responses in a manner that affects subsequent tissue repair.29–32
Several areas require further investigation. First, upstream regulators of QPRT need to be identified. We found that PGC1α induces QPRT expression, but the relevant transcriptional partner(s) remain elusive. Second, the present results suggest that renal and urinary quinolinate derive from impaired renal QPRT action, but this could also reflect renal accumulation from extrarenal sources that respond to renal ischemia. For example, QPRT’s expression is highest in the liver, where it should be studied further. Third, if QPRT is an important link between AKI and risk factors such as aging and chronic kidney disease, then one or more mechanisms implicated in aging and/or CKD may be important. Proposed mechanisms and effectors of aging include autophagy, redox balance, telomere shortening, epigenetic changes, and stem cell function.33 QPRT may be affected by or may impact several of these processes. Finally, the urinary metabolomic profiles of AKI and QPRT deficiency share 24 differentially regulated metabolites— approximately 40% of each profile. The non-overlapping metabolites may illuminate novel QPRT-independent metabolic stressors in AKI and/or actions of QPRT outside the scope of post-ischemic AKI.
The clinical studies propose both a new non-invasive indicator of NAD+ metabolism in the context of AKI and a novel set of targets for future therapeutic studies. Several important limitations should be noted. Because quinolinate is considered unique to the de novo NAD+ biosynthesis pathway, the evidence implicating uQ:T in clinical AKI strongly suggests that NAD+ metabolism is important in the human AKI context. While verification of this signal in a larger, prospectively collected ICU cohort helped to answer questions of confounding and over-fitting in the discovery study, future studies will need to address the timing and amplitude of uQ:T deflection in different AKI populations before considering this metabolite signature an AKI biomarker. Second, routine safety assessments of renal and cardiac function in the high-risk cardiac surgery population yielded signals suggesting benefit. But larger studies powered for these and related endpoints are clearly needed to address safety and efficacy. In this light, the present results provide valuable guidance for future trials. For example, since the 3 gm/day dose was well-tolerated and led to higher levels of circulating NMN than the 1 gm/day dose, this regimen may be more effective for increasing NAD+ metabolism. The severity of AKI was modest in our Phase 1 pilot study. Severe AKI after cardiac surgery is uncommon and would require much larger interventional studies to accumulate a sufficient number of severe events for analysis.34 Nonetheless, the incidence of AKI and the average post-operative rise in creatinine we observed in the placebo arm were comparable to large Phase 3 trials.34,35
Third, the optimal method to boost NAD+ remains to be determined. Although our choice of Nam was based upon its extensive human safety record, we also considered Nam’s ability to inhibit stress-activated PARPs as a possible adjunctive benefit.36 But Nam can also inhibit the cytoprotective enzyme sirtuin-1. Both Nam and another natural NAD+ precursor, nicotinamide riboside (NR), have been shown to increase intracellular NAD+; however, NR may also augment sirtuin activity more effectively than Nam.22 Complementary to orthogonal NAD+ supplementation, an add-on therapeutic strategy could also involve increasing flux through the de novo pathway—e.g., by administering de novo precursors or inhibiting enzyme(s) that deplete this pathway. Finally, compared to other metabolically active organs such as the brain or heart that are affected by aging or metabolic stressors, kidney function can be assessed quantitatively, reproducibly, and simply. Therefore, renal endpoints may become useful for future trials related to aging. More broadly, recognizing that age-dependent loss of resistance to acute metabolic stressors may be an aging phenotype suggests that exploratory interventional studies on aging could be conducted in a cost-efficient, acute context. In short, the biology of NAD+ should be examined in multiple clinical contexts.
To summarize, the present results demonstrate that impairment in de novo NAD+ biosynthesis characterizes patients at risk for AKI, a common and morbid complication of critical illness for which no specific treatment exists. Urinary measurement of de novo precursors in at-risk humans implicates impairment in this pathway and, furthermore, predicts adverse outcomes. The orthogonal NAD+ precursor Nam may be safe to administer among high-risk patients. Novel treatments to restore NAD+ could constitute an important advance for patients at risk of AKI. Further studies are needed to verify these findings.
ONLINE METHODS
Materials
All chemicals, except when noted, were purchased from Sigma-Aldrich, Saint Louis, MO.
MOUSE STUDIES
IRI model
All studies involving mice were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee (IACUC). Experiments were performed using littermate controls by an operator blinded to genotype and randomized within each cage to sham vs. AKI model. Creatinine from mouse serum was measured using LC/MS-MS at the University of Alabama Birmingham O’Brien Core Center for Acute Kidney Injury Research in a blinded fashion (NIH P30 DK079337). Bilateral renal ischemia-reperfusion injury (IRI) was performed as previously described.17 Nam or saline vehicle was administered as 400 mg/kg by intraperitoneal injections given 24 hours prior to IRI surgery, 1 hour prior to surgery, and 4–6 hours after surgery. Serum creatinine was measured in all IRI experiments at 24 hours after surgery.
CRISPR/Cas9 QPRT+/− mouse
Single guide RNAs (sgRNAs) were designed using the CRISPR guide-design application from MIT (crispr.mit.edu). All CRISPR reagents (Cas9 nickase and sgRNAs) were purchased from PNA Bio, Inc. Pronuclear stage zygotes were injected with CRISPR reagents and sgRNAs by the BIDMC Transgenic Core to generate founder mice on the C57BL6J background. Founder mice were verified by genotyping with the indicated primers in Supplemental Figure 2 and Supplemental Table 12. Male mice of 8–12 weeks of age were used in experiments.
Quantitative PCR
Total RNA extraction and cDNA synthesis were performed as previously described.17 PCR reactions were performed in duplicate using the ABI QuantStudio Flex 7 System (Applied Biosystems). SYBR primers were designed using PrimerQuest Tool (Integrated DNA Technologies) and sequences are in Supplemental Table 12. Relative expression levels were determined using the comparative threshold method.
Metabolomics measurements
Mouse urine and renal samples were analyzed in a blinded fashion with two distinct liquid chromatography-mass spectrometry (LC-MS) based methods. For positively charged polar analytes, 10 μL of urine was extracted with 90 μL of 74.9:24.9:0.2 vol/ vol/ vol acetonitrile/ methanol/ formic acid containing valine-d8 (Sigma-Aldrich; St Louis, MO). After centrifugation, 10 μL of supernatant underwent hydrophobic interaction chromatography using a 150 × 2.1 mm Atlantis hydrophobic interaction chromatography column (Waters, Milford, MA), and MS data were acquired on an Exactive Plus Orbitrap mass spectrometer (Thermo Scientific, Waltham, MA) using electrospray ionization in the positive ion mode. For negatively charged polar analytes, 20 μL of urine was extracted with the addition of 70 μL of 80:20 vol/vol methanol/water containing isotope-labeled inosine-15N4, 10 μL of supernatant underwent chromatography on a 150 × 2.0 mm Luna NH2 column (Phenomenex; Torrance, CA), and MS data were acquired on an Exactive Plus Orbitrap mass spectrometer (Thermo Scientific, Waltham, MA) using electrospray ionization in the negative ion mode. Data were processed using TraceFinder (v 3.0, Thermo Scientific, Waltham, MA) software, matching retention times and mass-to-charge ratio (m/z) to synthetic mixtures of reference compounds and characterized pooled plasma reference samples included in each sample queue.
NAD+ measurement
20–30mg of mouse kidney was homogenized with a Tissue-Tearor (Thomas Scientific, Swedesboro, NJ). Homogenization was performed in NAD+/NADH extraction buffer supplied with the NAD+/NADH Quantification Colorimetric Assay (Biovision, Milpitas, CA). 25μL of homogenate was assayed per manufacturer instructions and plate was read at 450nm using a 96-well plate reader (Molecular Devices, Sunnyvale, CA).
Histopathology
Formalin-fixed, paraffin-embedded blocks were sectioned and stained with hematoxylin and eosin and photographed with Axiovision for PC from Zeiss (Thornwood, NY).
Statistical considerations
Sample size for metabolomics experiments was based on previous experience.17 For IRI experiments focused on renal function (i.e., serum creatinine) outcome, sample size was based on previous experience and consideration of the following estimation: serum creatinine of 1.4 ± 0.5 mg/dl among controls vs. 2.1 ± 0.5 mg/dl among IRI mice requires n = 6 mice per group for 80% power and 5% α-error. For all mouse studies, non-parametric tests were used to compare continuous variables (Mann-Whitney U test or Kruskal Wallis if > 2 groups) unless otherwise noted. Data are presented as mean ± SEM unless otherwise specified. Results were prepared using Graphpad Prism Version 7 (La Jolla, CA). Two-tailed p-values < 0.05 were considered significant. *p<0.05, **p< 0.01, ***p<0.001, ****p< 0.0001 unless otherwise indicated.
HUMAN STUDIES
The human clinical studies conducted are described below as: (1) “uQ:T Discovery Nested Case-Control Study”; (2) “uQ:T ICU Validation Cohort”; (3) “Healthy Subject PK Study”; and (4) “Phase 1 Pilot Clinical Study of Oral Nam in Cardiac Surgery Patients”.
1. uQ:T Discovery Nested Case-Control Study
Study Population
We examined the uQ:T ratio as an indicator of risk for AKI in a prospective cohort of patients undergoing non-urgent, on-pump cardiac surgery through a nested case-control analysis. The prospective cohort for this nested case-control study was enrolled over a period of six months. The primary inclusion criterion was planned, on-pump cardiac surgery. Subjects were excluded for any of the following reasons: unresolved AKI 1 week prior to operation, history of kidney transplant, end stage renal disease, pregnancy, younger than 18 years of age, unable to consent, off-pump cardiac surgery, and individuals held in an institution by legal or official order. The study was in accordance with the Helsinki declaration, and approved by the institutional review board of Beth Israel Deaconess Medical Center (IRB 2010-P-000005/2). All subjects provided written informed consent prior to study enrollment. Study subjects were patients undergoing cardiac surgery at Beth Israel Deaconess Medical Center (Boston, MA). From January 2011 to June 2011 a total of 29 patients were enrolled (Supplemental Table 4), of which 6 had AKI. Using risk-set sampling,37 we randomly selected controls from the subgroup who had no AKI.
Assessment of AKI and other factors
AKI was defined by Kidney Disease Improving Global Outcomes (KDIGO) criteria for serum creatinine during any of the post-operative days 1 through 5.38 The first serum creatinine measured in each 24h calendar day was used as the daily value. Patients with AKI (n = 6) were matched with controls at a 1:1 ratio for age (± 11 years), ejection fraction (± 12%) based on echocardiogram results, aortic cross-clamp time (± 12 minutes) obtained from anesthesia records, and baseline creatinine (± 0.6 mg/dL) obtained from patient’s medical record and defined as most recent creatinine value prior to cardiac surgery. Assessment of other factors, including body mass index (BMI), gender, ethnicity, smoking history, clinical diagnosis of heart failure, hypertension, cardiopulmonary bypass time, valve surgery, and use of intra-aortic balloon pump was performed through review of the medical records. All subjects had complete clinical information.
Measurement of biochemical variables
Urinary samples were collected from subjects in standard polyethylene collection cups. Samples were collected the following time points: pre-operative, intra-operative, arrival to the ICU, 6 hours post-operative, and post-operative days 1 through 5. All samples were stored at −80C˚ and labeled with a unique identifier. Laboratory analysis for uQ:T ratio was performed by investigators (K.M.R. and A.H.B.) blinded to the exposure. Routine laboratory testing of human blood samples (creatinine, blood urea nitrogen, electrolytes, troponin T, liver function tests, C-reactive protein and complete blood count) was performed in CLIA (Clinical Laboratory Improvements Amendments)-certified clinical laboratories from the site at which the study was conducted.
Statistical analysis
Based on mouse data showing uQ:T of 1.1 ± 0.6 in controls vs. 2.8 ± 0.2 in mice with AKI (Fig 1d) and assuming a comparable difference in humans, examining 6 controls vs. 6 AKI-affected subjects was estimated to provide 85% power to detect a between-group difference with α-error 0.05. To further analyze for temporal trends between cases and controls in uQ:T, we used two-factor ANOVA. No imputation was performed. All P values are two-tailed, and P values below 0.05 were considered to indicate statistical significance. All analyses were performed using Prism 7 (GraphPad Software Inc.).
2. uQ:T ICU Validation Cohort
Study Population
The ICU validation cohort included subjects admitted to the medical or surgical intensive care unit (ICU) at Brigham and Women’s Hospital (BWH; Boston, MA) between September 2008 and January 2013. Further details of this cohort have been published.19 The study was performed in accordance with the Declaration of Helsinki and was approved by the Partners Human Research Committee (IRB protocol #2007P000894). All patients provided written and informed consent.
Assessment of AKI and other factors
The primary end point was incident AKI, as defined by changes in serum creatinine (urine output data were not available) in accordance with the KDIGO criteria.39 The highest serum creatinine value from each 24h calendar day was recorded and used for assessment of AKI. To maintain the prospective nature of the study, incident AKI was defined as new AKI occurring within 7 days after enrollment. Accordingly, patients who already had AKI at the time of enrollment were excluded from these analyses (Supplemental Figure 5). Secondary end points included a composite of incident AKI or in-hospital mortality (AKI/death), severe AKI, RRT or in-hospital mortality (RRT/death), and death (assessed in-hospital and at 1 year). Severe AKI was defined as doubling of serum creatinine or need for RRT, corresponding to stages 2 and 3 of the KDIGO criteria.
Measurement of biochemical variables
Urinary samples were collected from subjects in standard polyethylene collection cups. Samples were collected within 48h of arrival to the ICU, and then daily thereafter for 5 days. All samples were stored at −80C˚ and labeled with a unique identifier. Laboratory analysis for uQ:T ratio was performed by investigators (K.M.R. and A.H.B.) blinded to the exposure. Serum creatinine was measured for routine clinical purposes by the central clinical laboratory at BWH.
Statistical analysis
A post hoc sample size calculation for the ICU cohort study demonstrated that 215 patients with an incident AKI event rate of 24% (n = 51) provided > 80% power to detect an odds ratio of 1.7 per standard deviation of uQ:T. All P values are two-tailed, and P values below 0.05 were considered to be statistically significant. All analyses were performed using SAS Version 9.4 (Cary, NC). Covariates included in multivariable models consisted of both comorbidities (e.g., hypertension and diabetes mellitus) and severity of illness factors. Comorbidities were ascertained by manual chart review. Specifically, patients were considered to have these comorbidities if the comorbidities were listed under “Past Medical History” in their chart. Severity of illness factors included need for mechanical ventilation, which was assessed by manual chart review on enrollment, ICU type (dichotomized as medical versus surgical), and the Acute Physiology and Chronic Health Evaluation (APACHE) II score, which is a severity of illness scoring system ranging from 0 to 71, with higher score indicating more severe disease.39 Comparison of metabolite levels between those with AKI and those without AKI was performed by Wilcoxon Rank Sum test. Multivariate logistic regression was used to assess the association between uQ:T and adverse outcomes with adjustments for covariates as described above. Ten subjects had missing APACHE II scores in the dataset. All other clinical data points, and biospecimens were complete for all patients. No imputation for missing data was performed.
3. Healthy Subject PK Study
Study Population
Based upon a comparably-sized previous study of oral Nam pharmacokinetics in healthy volunteers,23 nine participants were consented. One subject withdrew from the study following a new diagnosis of a chronic medical condition. The remaining eight, who were free of chronic illness, were given oral Nam 3 gm once daily supplied by the Massachusetts General Hospital research pharmacy (Rugby Laboratories) at 0, 24 and 48 hours between January 2016 to June 2016. Eligible subjects were identified through flyers posted throughout the Massachusetts General Hospital campus, and online on the Partners Healthcare Clinical Trials in need of Volunteers Website (https://clinicaltrials.partners.org/). Subjects who completed the study were remunerated with a check for $120. All patients provided written and informed consent. The study was conducted at the Massachusetts General Hospital and approved by the Institutional Review board (MGH IRB 2015P0019). The study was performed in accordance with the Declaration of Helsinki.
Assessment of Adverse Events and serum Nam levels
The primary outcome was defined as the temporal trend in serum Nam levels. Secondary outcomes included serum creatinine values and adverse events. Adverse events were measured using a standard questionnaire and self-reporting of any symptoms after Nam administration. Blood samples for serum Nam and creatinine were obtained at hours 0, 1, 2, 3, 5 hours on the first day and 2 hours after the oral Nam dose on days two and three. Additionally, a blood sample was collected 24 hours after the last Nam dose.
Measurement of creatinine and Nam levels
Laboratory testing for creatinine was performed in CLIA-certified clinical laboratories from the site at which the study was conducted and confirmed through LC-MS analyses performed using an API 5000 triple quadrupole mass spectrometer from AB Sciex (Foster City, CA) coupled to a Shimadzu Prominence UFLC liquid chromatography system with autosampler (Shimadzu Scientific Instruments, Colombia, MD).
Statistical analysis
Within-subject serum creatinine levels over time were compared to baseline through paired t-test. All P values are two-tailed, and P values below 0.05 were considered to indicate statistical significance. All analyses were performed using Prism 7 (GraphPad Software Inc.).
4. Phase 1 Pilot study of Oral Nam in Cardiac Surgery Patients
Study Population
In this phase 1 pilot randomized, single blind, placebo-controlled clinical trial, patients were identified and screened through surgical appointment logs and electronic medical records. Patients were approached at their outpatient preadmission evaluation or on inpatient hospital wards, at which time the study explained and written and signed informed consent was obtained prior to enrollment. The trial was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center (IRB 2016P000028) and registered at clinicaltrials.gov (NCT02701127). The study was conducted according to the principles of the Declaration of Helsinki. The inclusion criterion for this study was planned on-pump cardiopulmonary bypass surgery. An additional inclusion criterion of Cleveland Clinic Score ≥ 6 (Supplemental Table 9) was removed through IRB-protocol amendment due to lack of sufficient patients meeting this criterion. Subjects were excluded for any of the following reasons: any new AKI within 1 week prior to operation, kidney transplant status, end stage renal disease, pregnancy, younger than 18 years of age, inability to consent, off-pump cardiac surgery, and individuals held in an institution by legal or official order. No patients with urgent cases were enrolled.
Randomization and Blinding
Blocked randomization schedules were electronically generated with a block size of 6 and maintained by the Beth Israel Deaconess Medical Center research pharmacy, which was not involved in patient care or data analysis. Patients were randomly assigned on a 1:1:1 basis into the three study arms (1 gm/d Nam, 2 gm/d Nam, or placebo). Patients were stratified according to history of CKD, defined as a history of proteinuria (dipstick ≥ 1+) or eGFR <45 ml/min prior to enrollment. Screening and enrollment took place between July 2016 and January 2017. Randomization was concealed and carried out by the research pharmacy at Beth Israel Deaconess Medical Center. Patients, cardiac surgeons, and other members of the healthcare team, as well as the investigators analyzing molecular markers (K.M.R. and A.H.B.), were blinded to treatment assignment. Placebo and Nam (Rugby Laboratories) were supplied by the Beth Israel Deaconess Medical Center research pharmacy.
Assessment of primary and secondary endpoints and adverse events
The primary end-point in this phase 1 pilot trial was the change of serum Nam in the setting of high dose oral Nam administration among patients undergoing on-pump cardiac surgery. Secondary endpoints were changes in urinary Nam concentration and assessment for adverse events. Per specific guidance from the IRB prior to study approval regarding the perioperative risks related to cardiac surgery, laboratory safety assessments included cardiac enzymes, renal function tests, and liver biochemistries. We also evaluated length of ICU stay, length of hospital stay, 30-day re-hospitalization, and all-cause mortality. A detailed schedule of assessments is included (Supplemental Table 10). Blood and urine samples for NAD+ metabolite measurements were obtained as part of routine clinical care. Blood samples to determine NAD+ metabolites were successfully collected for 82% (placebo), 71% (1 gm/d arm), and 72% (3 gm/d arm) of the prespecified schedule of assessments. > 95% of collected specimens were evaluated successfully for NAD+ metabolites. Unanticipated adverse events were identified through subject interview, communication with the clinical team, and through manual chart review. Safety data were monitored on an ongoing basis. The principal investigator (A.P.M.) and co-investigator (V.W.) monitored all subjects matriculating through the trial for signs of toxicity and other adverse events. Adverse events including, but not limited to, cardiovascular events and abnormal laboratory findings were reviewed, adjudicated, and shared with an independent medical monitor (SJH) for review. All serious adverse events were reported to the IRB. For primary and secondary endpoints, all patients had complete clinical data. No imputation of the data was performed. Study data were collected and managed using REDCap electronic data capture tools hosted at Beth Israel Deaconess Medical Center.40 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Blood and urine sampling and analysis
Baseline blood and urine samples were drawn at the pre-op evaluation visit if the study participant was outpatient, or the day prior to surgery if the participant was already hospitalized. Subsequent follow up assessments were performed immediately after surgery and on post-operative days 1 through 3. All samples were stored at −80C˚ and labeled with a unique identifier. Routine laboratory testing of human blood samples (creatinine, blood urea nitrogen, electrolytes, troponin T, liver function tests, C-reactive protein and complete blood count) was performed in CLIA-certified clinical laboratories from the site at which the study was conducted. The first serum creatinine and troponin T measured in each 24h calendar day were used as the daily value. The definition of AKI was adopted from KDIGO as a rise in serum creatinine ≥ 0.3 mg/dL and assessed at any of the post-operative days 1 through 3. This definition was applied in a recent Phase 3 trial of AKI (see Supplemental Table 11).8
Statistical Analysis
We estimated that 5 subjects per group would provide >95% power to detect differences in serum Nam concentration between the 3 gm/d Nam and placebo groups with α-error 1%. However, based on recent literature describing an AKI event rate of ~40%,34 we estimated a sample size of 10–15 patients per group for this Phase 1 study (each yielding approximately 5 patients with AKI) would be sufficient to assess pharmacokinetic effects of oral Nam in on-pump cardiac surgery patients with and without AKI. To account for screen failures and loss to follow-up, we set the target enrollment for 20 patients per group. The primary and secondary endpoint analyses included all patients who were randomized and matriculated through the study according to the protocol. Descriptive statistics are summarized for categorical variables as frequency (%), for normally distributed continuous variables with means (±SD), and for skewed continuous data with median and interquartile range (IQR). For categorical data, between-group comparisons were conducted with chi-square test. Normally distributed continuous data were compared with unpaired t test. Skewed continuous data were analyzed with non-parametric tests (Mann-Whitney-U tests for unpaired, in-between group, and Wilcoxon for paired, within group observations). No interim analysis was performed. The primary endpoint analysis compared serum profiles of Nam among the 3 different groups of 1 gm/d, 3 gm/d, and placebo using areas under the curve (AUCs) against placebo in accordance with the analyses reported by Trammell, et al.22 All P values are two-tailed, and P values below 0.05 were considered to indicate statistical significance. All analyses were performed using Prism 7 (GraphPad Software Inc.).
Human metabolite measurements
Isotopic standards
Isotopic standards (tryptophan-d5, nicotinamide-d4, 13C-creatinine) were purchased from Toronto Research Chemicals (Toronto, Canada). Isotopic standards were mixed at a concentration of 50 μM and mixed 1:1 with urine and serum samples prior to protein precipitation with acetonitrile (80% w/v final concentration).
Instrumentation
All LC-MS analyses were performed using an API 5000 triple quadrupole mass spectrometer from AB Sciex (Foster City, CA) coupled to a Shimadzu Prominence UFLC liquid chromatography system with autosampler (Shimadzu Scientific Instruments, Colombia, MD). High performance chromatography of both peptides and amino acids was performed using hydrophilic interaction chromatography on Luna HILIC UPLC columns (100 × 3.0 mm, 2.6 μm bead diameter, 100A pore size, Phenomenex, Inc., Torrance, CA). Instrument control, data acquisition and quantification were performed using Analyst 1.6.2 software (Sciex, Framingham, MA).
Serum and urine small molecule analyses
10 μL of serum or urine were mixed with 10 μL isotopic standards (50 micromoles/L each) diluted in water. Serum/urine proteins were then precipitated by mixing with 80 μL acetonitrile (80% final conc.), and precipitates were cleared by centrifuging at 14,000 rpm for 10 minutes. Supernatant was transferred into a 96-well plate for analysis. Analysis was performed by hydrophilic interaction chromatography on a Luna HILIC column using a gradient elution protocol. Chromatograms of the extracted ion MRM metabolite peaks from a representative urine sample are shown in Supplemental Figure 4. Calibration standards for each metabolite were purchased from Sigma Aldrich, weighed and diluted into phosphate buffered saline at 100 mM, and calibrator mixtures were made from stock solutions starting at 100 μM. Coefficient of variation for individual metabolites was 4–7%.
The data that support the findings of this study are available from the corresponding author upon reasonable request. For any patient-related information, data requests will be addressed in consultation with the Institutional Review Board overseeing the study.
Supplementary Material
Supplemental Table 1: List of metabolites measured in mouse urine
Supplemental Table 2: List of differentially regulated urinary metabolites in AKI vs. control urines
Supplemental Table 3: List of differentially regulated urinary metabolites in QPRT+/− vs. WT
Supplemental Table 4: Baseline characteristics for uQ:T discovery cohort
Supplemental Table 5: Characteristics of cases vs. controls for uQ:T discovery study
Supplemental Table 6: Baseline characteristics for uQ:T ICU validation cohort
Supplemental Table 7: uQ:T (urinary quinolinate/tryptophan) ratio and incident AKI and other adverse outcomes in critically ill patients
Supplemental Table 8: Baseline characteristics of healthy subject oral Nam PK study
Supplemental Table 9: Cleveland Clinic Foundation Cardiac Surgery Risk Score
Supplemental Table 10: Schedule of assessments for Phase 1 pilot study of oral Nam in cardiac surgery patients
Supplemental Table 11: Phase 1 pilot study of oral Nam in cardiac surgery patients peri-operative AKI definition
Supplemental Table 12: Primer and single guide RNA (sgRNA) sequences
Supplemental Figure 1: QPRT expression among organs, within kidney, and in response to PGC1α
Supplemental Figure 2: Development of QPRT-deficient mouse.
Supplemental Figure 3: Flow of participants through uQ:T discovery study
Supplemental Figure 4: Development of targeted metabolite assays
Supplemental Figure 5: Flow of participants through uQ:T ICU validation study
Supplemental Figure 6: Serum Nam and creatinine in healthy subject oral Nam PK study
Supplemental Figure 7: AKI events in Phase 1 pilot study of oral Nam in cardiac surgery patients
Acknowledgments
The authors thank members of the Cardiovascular Institute and Division of Cardiac Anesthesia at Beth Israel Deaconess Medical Center for support of the Phase 1 pilot study of oral Nam in cardiac surgery patients. This randomized clinical trial was funded by an Innovation Grant from Beth Israel Deaconess Medical Center awarded to A.P.M. and K.R.K. K.M.R., V.W., A.K., and M.R.L. were supported by T32DK007199; D.E.L. by K23DK106448; C.C.K. and R.I.T. by T32DK007540; S.J.H. by K23AG042459; N.S.-T. by a grant from Assistance Publique—Hospitaux de Paris; A.H.B. by K08HL121801 and R56HL133399. Work in S.M.P.’s laboratory was supported by R35HL139424, R01HL125275, and R01DK095072.
Footnotes
AUTHOR CONTRIBUTIONS
A.P.M. and K.R.K. were co-PIs on the Phase 1 pilot study of oral Nam in cardiac surgery patients for which V.W., J.M., A.L., and M.E.T. were co-investigators. S.J.H. served as the independent medical monitor for this trial. K.M.R., N.S.-T., and A.H.B. analyzed samples and data from all human studies. A.H.B. developed targeted metabolic assays and conducted all related measurements. M.T.T. conducted mouse studies and analyzed results with assistance from N.S.-T. and M.R.L. D.E.L. and S.S.W. enrolled the ICU cohort, created that repository, and performed statistical analyses of the uQ:T results in the ICU cohort. A.K. and S.H.K. enrolled the discovery cohort of cardiac surgery patients and created that repository with guidance from S.M.P. C.C.K., E.P.R., and R.I.T. developed and conducted the trial of oral Nam in healthy volunteers. E.P.R. also conducted metabolomic screening of mouse samples on the platform developed in C.B.C.’s laboratory with input from C.B.C. A.P.M., M.T.T., K.M.R., and S.M.P. assumed primary responsibility for writing the manuscript. All authors reviewed, provided substantive input, and approved of the final manuscript.
- S.M.P. is listed as an inventor on disclosures filed by Beth Israel Deaconess Medical Center pertaining to NAD+.
References
- 1.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nature reviews Molecular cell biology. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell metabolism. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ternes CM, Schonknecht G. Gene transfers shaped the evolution of de novo NAD+ biosynthesis in eukaryotes. Genome Biol Evol. 2014;6:2335–2349. doi: 10.1093/gbe/evu185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. The Journal of biological chemistry. 1972;247:778–783. [PubMed] [Google Scholar]
- 5.Krehl WA, Teply LJ, Sarma PS, Elvehjem CA. Growth-Retarding Effect of Corn in Nicotinic Acid-Low Rations and Its Counteraction by Tryptophane. Science. 1945;101:489–490. doi: 10.1126/science.101.2628.489. [DOI] [PubMed] [Google Scholar]
- 6.Kleta R, et al. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nature genetics. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- 7.Canto C, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G, et al. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158:1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams PA, et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–760. doi: 10.1126/science.aal0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes AP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016 doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 13.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 14.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 15.Mills KF, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta RL, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 17.Tran MT, et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney international. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leaf DE, et al. Fibroblast Growth Factor 23 Levels Associate with AKI and Death in Critical Illness. Journal of the American Society of Nephrology: JASN. 2017;28:1877–1885. doi: 10.1681/ASN.2016080836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 21.Chen AC, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. The New England journal of medicine. 2015;373:1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 22.Trammell SA, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nature communications. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petley A, Macklin B, Renwick AG, Wilkin TJ. The pharmacokinetics of nicotinamide in humans and rodents. Diabetes. 1995;44:152–155. doi: 10.2337/diab.44.2.152. [DOI] [PubMed] [Google Scholar]
- 24.Bai P, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell metabolism. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morigi M, et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015;125:715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agledal L, Niere M, Ziegler M. The phosphate makes a difference: cellular functions of NADP. Redox Rep. 2010;15:2–10. doi: 10.1179/174329210X12650506623122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkland JB. Niacin requirements for genomic stability. Mutat Res. 2012;733:14–20. doi: 10.1016/j.mrfmmm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Shi H, et al. NAD Deficiency, Congenital Malformations, and Niacin Supplementation. The New England journal of medicine. 2017;377:544–552. doi: 10.1056/NEJMoa1616361. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16:279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opitz CA, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 31.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357 doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 33.Underwood E. The final countdown. Science. 2015;350:1188–1190. doi: 10.1126/science.350.6265.1188. [DOI] [PubMed] [Google Scholar]
- 34.Hausenloy DJ, et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. The New England journal of medicine. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 35.Garg AX, et al. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA. 2014;311:2191–2198. doi: 10.1001/jama.2014.4952. [DOI] [PubMed] [Google Scholar]
- 36.Ebrahimkhani MR, et al. Aag-initiated base excision repair promotes ischemia reperfusion injury in liver, brain, and kidney. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4878–4886. doi: 10.1073/pnas.1413582111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langholz B, Goldstein L. Risk set sampling in epidemiologic cohort studies. Statistical Science. 1996;11:35–53. [Google Scholar]
- 38.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney international. 2012;(Suppl):1–138. [Google Scholar]
- 39.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 40.Harris PA, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: List of metabolites measured in mouse urine
Supplemental Table 2: List of differentially regulated urinary metabolites in AKI vs. control urines
Supplemental Table 3: List of differentially regulated urinary metabolites in QPRT+/− vs. WT
Supplemental Table 4: Baseline characteristics for uQ:T discovery cohort
Supplemental Table 5: Characteristics of cases vs. controls for uQ:T discovery study
Supplemental Table 6: Baseline characteristics for uQ:T ICU validation cohort
Supplemental Table 7: uQ:T (urinary quinolinate/tryptophan) ratio and incident AKI and other adverse outcomes in critically ill patients
Supplemental Table 8: Baseline characteristics of healthy subject oral Nam PK study
Supplemental Table 9: Cleveland Clinic Foundation Cardiac Surgery Risk Score
Supplemental Table 10: Schedule of assessments for Phase 1 pilot study of oral Nam in cardiac surgery patients
Supplemental Table 11: Phase 1 pilot study of oral Nam in cardiac surgery patients peri-operative AKI definition
Supplemental Table 12: Primer and single guide RNA (sgRNA) sequences
Supplemental Figure 1: QPRT expression among organs, within kidney, and in response to PGC1α
Supplemental Figure 2: Development of QPRT-deficient mouse.
Supplemental Figure 3: Flow of participants through uQ:T discovery study
Supplemental Figure 4: Development of targeted metabolite assays
Supplemental Figure 5: Flow of participants through uQ:T ICU validation study
Supplemental Figure 6: Serum Nam and creatinine in healthy subject oral Nam PK study
Supplemental Figure 7: AKI events in Phase 1 pilot study of oral Nam in cardiac surgery patients