Abstract
Objectives
This study examined the differences in cognitive function between middle-aged and older adults with insomnia disorder, insomnia symptoms only (ISO) or no insomnia symptoms (NIS), in the context of other health and lifestyle factors.
Methods
Twenty-eight thousand four hundred eighty-five participants >45 years completed questionnaires, physical examinations, and neuropsychological testing across domains of processing speed, memory, and executive functions. An eight-question instrument assessed participants’ sleep, defining subjects with insomnia symptoms, probable insomnia disorder (PID), or NIS. The associations between these three groups and cognitive performance were examined with linear regression models adjusted for lifestyle and clinical factors.
Results
PID was identified in 1,068 participants (3.7% of the sample) while 7,813 (27.5%) experienced ISO. Participants with PID exhibited greater proportions of adverse medical and lifestyle features such as anxiety, depression, and diabetes than both other groups. Analyses adjusting for age, sex, education, as well as medical and lifestyle factors demonstrated that adults with PID exhibited declarative memory deficits (Rey Auditory Verbal Learning Test) compared with ISO or NIS. Adults with insomnia symptoms exhibited better performance on a task of mental flexibility than both other groups.
Conclusions
These findings suggest that insomnia disorder in middle-aged and older adults is associated with poorer health outcomes and worse memory performance than adults with insomnia symptoms alone or without any sleep complaints, even after adjustment for comorbidities. The assessment of longitudinal data within this cohort will be critical to understand if insomnia disorder may increase the risk of further cognitive decline.
Keywords: insomnia, cognition, aging, cohort, CLSA
Statement of Significance.
This study utilizes a large dataset acquired from the Canadian Longitudinal Study on Aging to compare the prevalence, clinical characteristics, and cognitive function of middle-aged and older adults with probable insomnia disorder (PID). Most previous large-sample epidemiological studies in this area have been limited by the inability to utilize clinical diagnosis of insomnia disorder at such scale and have instead focused on broad sleep measures (e.g., sleep duration, overall subjective sleep quality) or insomnia symptoms only (ISO). Indeed, compared with other adults who experience ISO (but do not meet criteria for insomnia disorder) or no insomnia symptoms at all, PID was associated with a range of poorer lifestyle and medical outcomes, as well as impaired performances on a range of cognitive tests. After controlling for these lifestyle and medical factors, memory performances remained significantly worse in adults with PID, highlighting the presence of poorer memory function in middle-aged and older individuals with insomnia disorder. This study will help to direct future analyses investigating whether insomnia disorder increases the risk of further cognitive decline longitudinally with age.
Introduction
Insomnia is one of the most common sleep disorders, estimated to affect approximately 9%–10% of adults in the general population [1, 2]. A diagnosis of insomnia disorder is made when an individual has trouble falling asleep, staying asleep, or experiences early morning awakenings more than three times per week, for longer than 3 months, accompanied by a subjective experience of poor sleep quality and daytime dysfunction [3]. Meanwhile, an additional 25%–30% of adults may occasionally experience insomnia symptoms that do not reach the full spectrum of an insomnia disorder diagnosis, for example with no reported impact on daytime function [1, 2]. Given that sleep is implicated in multiple beneficial roles (e.g. within cardiovascular [4], immune [5], and neural [6] systems), it is important to distinguish between insomnia symptoms and insomnia disorder to assess whether a full insomnia disorder phenotype is required to impact these processes leading to chronic health conditions.
Research has shown that insomnia disorder is often comorbid with psychiatric disorders such as depression and anxiety [7], cardiovascular disease [8], diabetes [9], and other sleep disorders such as obstructive sleep apnea [10]. Insomnia disorder has also been linked to impairments in cognitive function across a range of domains, yet studies investigating cognitive performances in clinical samples with insomnia disorder have produced mixed results, partly due to limited sample sizes, and considerable variability in study characteristics (e.g. selection criteria and assessment methods) or unmeasured confounding variables such as comordbidities [11–16].
On the other hand, most large-sample epidemiological studies in this area have been limited by the inability to utilize clinical diagnosis of insomnia disorder at such scale and have instead focused on broad sleep measures (e.g., sleep duration, overall subjective sleep quality) or insomnia symptoms only (ISO) [17–22]. There have only been two cohort studies that have specifically investigated probable insomnia disorder (PID) and cognitive function [23, 24]. Neither study observed any difference in cognitive function between insomnia and controls overall. However, both found that specific subgroups of insomnia exhibited cognitive deficits. One study included a representative sample of 1,037 adults all aged 38 years who had been followed from birth. This study found that of those meeting DSM-IV inclusion criteria for insomnia, only those who had also sought treatment for insomnia (defined as discussing insomnia with a health professional) exhibited deficits in tests of processing speed and verbal comprehension [23]. The other study, using a more general inclusion criterion of a complaint of insomnia with a duration ≥1 year from a randomly selected cohort of 678 adults, detected impaired performance in processing speed and attention tasks only among participants who displayed short sleep duration, as objectively defined by a threshold of less than 6 h total sleep time [24]. Taken together, this suggests that more severe phenotypes of insomnia may include cognitive impairment, however the distinction between insomnia disorder and insomnia symptoms regarding their effects on cognitive function has not been examined, particularly in older adults. Furthermore, given the range of comorbidities often concomitant with insomnia disorder, certain factors may place individuals with insomnia disorder at a greater risk of cognitive impairment. However, controlling for the effects of comorbidities and considering phenotype when assessing the association between insomnia and cognitive function has yet to be performed in a systematic way within a large sample.
Thus, the aim of this study was to determine whether an association exists between the presence of PID and deficits across various cognitive domains in a large sample of middle-aged and older adults; distinct from ISO and independent of relevant demographic, lifestyle, and medical factors. This was addressed by utilizing the baseline data of a large-scale cohort study. We hypothesized that we would observe poorer cognitive function in adults with insomnia symptoms and insomnia disorder, compared with those without insomnia, but that these deficits would be more severe in insomnia disorder, even after accounting for medical comorbidities and lifestyle factors.
Methods
Study design and participants
The sample was sourced from the 30,097 participants who completed the baseline assessments of the “comprehensive cohort” from the Canadian Longitudinal Study on Aging (CLSA). The CLSA is a national, 20-year, prospective cohort study, and the CLSA study design and recruitment process has been comprehensively described and published elsewhere [25]. This large-scale cohort study collects information on the biological, medical, cognitive, psychological, social, lifestyle, and economic aspects of middle-aged and older adults. Briefly, individuals were recruited through either health registration databases or random digit dialing. Eligible participants were required to be aged greater than 45 years, live within 25 or 50 km of 1 of 11 data collection sites (depending on the site), able to respond in English or French, and exhibit an absence of cognitive impairment at the time of testing through standardized cognitive screeners. For this particular study, further exclusion criteria included: any prior diagnosis of dementia, stroke, or major head injury resulting in a loss of consciousness >20 minutes. The final sample consisted of 28,485 individuals who met the criteria for this study.
Study participants who are part of the CLSA comprehensive cohort were asked to provide information through questionnaires, physical examinations, neuropsychological battery, and biological samples—administered per standardized protocols. Questionnaires were administered during an in-home interview. Neuropsychological data were collected during visits to data collection centers for the vast majority of participants. At-home visits were conducted when participants were unable to travel to the data collection center (0.74%).
Sleep and insomnia measures
Measures of sleep habits were collected through structured self-report questions, that were designed for this study as part of a larger set of measures to assess physical functioning. The questions were drawn from a variety of sources [26–28] and captured important aspects of sleep and their relation to health. The questions covered six domains: (1) participants’ satisfaction with the type of sleep they were getting; (2) their reported total amount of sleep during the night (“During the past month on average how many hours of actual sleep did you get at night?”); (3) whether they were having trouble falling asleep or staying asleep; (4) whether they had trouble staying awake when they did not intend to sleep; (5) if they reported or had ever been told that they seemed to “act out their dreams” or move while sleeping, and; (6) if they experienced, recurrent, uncomfortable feelings or sensations in the legs, or urges to move their legs while sitting or lying down.
Symptoms related to insomnia were drawn from domain 3. Specifically, these were assessed via the questions: “Over the last month, how often did it take you more than 30 minutes to fall asleep?” and “Over the last month, how often did you wake in the middle of the night or too early in the morning and found it difficult to fall asleep again?” Possible responses were: never, less than once per week, once or twice a week, 3–5 times/week, or 6–7 times/week. For each insomnia symptom, participants were also asked: “To what extent do you consider your problem to interfere with your daily functioning (for example, from daytime fatigue, ability to function at work/daily chores, concentration, memory, mood, etc.)?” Possible responses were: not at all, a little, somewhat, much, or very much. They were also asked: “For how long have you had this trouble?” Finally, participants were asked: “How satisfied or dissatisfied are you with your current sleep pattern?” Possible responses were: very satisfied, satisfied, neutral, dissatisfied, very dissatisfied.
These questions were utilized to make a probable diagnosis of insomnia disorder based on standard diagnostic criteria from the DSM-5 [3] (Supplementary Figure 1). Specifically, only participants who experienced difficulties with sleep onset or maintenance more than three times per week, for longer than 3 months, and additionally stated that it significantly interfered with their daily functioning were categorized as having PID. Any participant who experienced these symptoms with the same frequency but did not report any interference with daytime functioning were categorized as having ISO. All other participants were classified as having no insomnia symptoms (NIS). The specific classification of PID and ISO based on these questions is provided in Table 1, and further information on the criteria used in the classification is provided in Supplementary Figure 2.
Table 1.
Criteria used for categorization into PID and ISO
| Criteria for PID | Criteria for ISO |
|---|---|
| Sleep onset >3× per week OR Sleep maintenance >3× per week AND Interferes with daytime function ≥ “Much” AND Has been present >3 months AND Satisfaction with sleep quality < “Neutral” |
Sleep onset >3× per week OR Sleep maintenance >3× per week AND Interferes with daytime function < “Much” |
Cognitive performance measures
Neuropsychological testing was administered to participants by a trained interviewer. Participants completed tests that assessed performance across three broad cognitive domains: memory, executive functions, and psychomotor speed using well-validated measures.
Declarative memory was assessed with the Rey Auditory Verbal Learning Test (RAVLT), a 15-item word learning test that assesses both learning and retention [29]. The number of words recalled in one immediate recall trial (RAVLT I) and one delayed recall trial (RAVLT II, with a delay of 30 min) were reported.
Executive functions were assessed using the Mental Alternation Test (MAT) [30], Stroop Test (Victoria version) [31], Controlled Oral Word Association Test (COWAT) [32], and Animal Fluency Test (AFT) [30]. The MAT, is a measure of mental flexibility and processing speed, requiring participants to count aloud from 1 to 20 and say the alphabet in alternation between number and letter (i.e. 1-A, 2-B, 3-C, …) as quickly as possible for 30 s, with the number of items attained being the outcome. The Stroop test is a measure of mental speed, inhibition, attention, and mental control and has three parts. In the first part (Stroop 1), the participant was asked to read a list of words printed in different ink colors, and in the second part (Stroop 2), to name the ink color of printed symbols (X’s). In the third part (Stroop 3, interference condition), names of colors were printed in mismatching colored ink, and the participant was asked to quickly name the color of the ink that the words are written in and not to read the words (e.g. say “blue” for the word “green” written in blue ink). For all parts of the Stroop test, the outcome was the time taken to complete the task. The number of errors on Part 3 of the Stroop test was also analyzed separately. The COWAT is a measure of phonological fluency or knowledge, where the participant was asked to list out as many words as possible that begin with a certain letter over three 60-s trials (using a different letter each trial, e.g. F, A then S). The AFT is a similar test of category fluency, where participants were asked to list as many names of animals as possible in one 60-s trial.
Psychomotor speed was assessed with the choice reaction time (CRT) test, in which participants were instructed to touch an interactive computer screen as quickly as possible at the location of a box that appears following a 1,000 ms delay. All tests were scored in a standardized way in collaboration with a CLSA co-investigator who was a clinical neuropsychologist.
Demographic and lifestyle measures
Sociodemographic information, including age, sex, household income and years of formal education, was collected. The interview and questionnaire also included items measuring tobacco consumption (current and former smoking habits), as well as items measuring the amount of alcohol consumed per typical week over the past 12 months.
Medical measures
Participants self-reported whether a doctor had ever told them that they had any of a range of chronic conditions, including hypertension, neurological, anxiety, depression, cancer, and chronic pain. Self-reported, clinician-diagnosed, chronic conditions have been shown to have high test–retest reliability in population-based health surveys [25]. Medical comorbidities were not an exclusion criteria. This analytic decision enabled us conduct statistical models investigating their impact on the relationship between insomnia and cognitive function. Trained CLSA staff also measured the height and weight of participants, to calculate body mass index (BMI). Patients also reported how often they found it difficult to stay awake during normal waking hours or whether anyone ever had observed them stop breathing in their sleep.
Statistical analysis
Differences in demographic and clinical characteristics between PID, ISO, and NIS were assessed using ANOVA with Tukey’s honest significance test, or Pearson’s chi-squared tests. The association between the three groups and cognitive function expressed continuously was examined with general linear models using the “lm” and “Anova” functions of the “stats” package in R [33]. For each cognitive outcome, three multiple regression models were created with increasing complexity. A minimally adjusted model (model 1) adjusted for age, sex, total household income, and years of education. The next model (model 2) additionally adjusted for a range of a priori factors that were considered relevant in the context of cognitive aging. These factors included: BMI, weekly level of alcohol consumption, diagnosis of cancer, diagnosis of anxiety disorder or clinical depression, hypertension, current level of smoking, presence of chronic pain, and a report of daytime sleepiness and a witness report of breathing interruption during sleep (to provide a proxy for sleep apnea). The final model (model 3) adjusted for everything in model 2, in addition to a binary variable indicating whether each subject regularly ≥6 h, or <6 h per night. This was chosen based upon previous findings that only insomnia disorder with <6 h sleep exhibit cognitive impairment [24]. All results are presented as adjusted least-squares means and 95% confidence intervals. A false discovery rate correction [34] to account for multiple comparisons was applied, separately for the group-level comparison of demographic and clinical characteristics, and for each regression model between insomnia group and all cognitive tests. While this approach is less conservative than other methods, type II errors are less likely, and the approach provides better control against errors originating from multiplicity.
To determine variables and interactions that significantly predicted worse cognitive performance within each group, decision tree regression was performed using the “rpart” package within R [35]. The “rpart” package implements the Classification and Regression Trees (CART) algorithm [36]. Linear regression tree models were applied post-hoc for each cognitive outcome that were found to be significantly associated with PID in the general linear models. The variables included for decision making in the decision tree regression were all covariates that were implemented in model 3. The number of cross validations was set to 1,000, and the minimum number of instances allowed in a node prior to a split was set to 100 (10%). The complexity parameter was set to 0 and the resulting trees were subsequently pruned to the node with the lowest x-error.
Results
Sample characteristics
The analysis cohort was comprised of 28,485 adults with a mean age 62.8 ± 10.2 years. One-quarter (25.8%, n = 7,337) were aged between 45 and 54 years, 9,379 (32.9%) between 55 and 64 years, 6,927 (24.3%) between 65 and 74 years, and 4,842 (17.0%) were aged more than 75 years. Slightly more than half (51.6%, n = 14,686) were females, 24,258 (85.2%) had more than 12 years of education and 95.6% were Caucasian. English was the primary language for 22,369 (78.5%) participants, while 5,390 (18.9%) spoke primarily French and 2.6% had another native language.
Sleep duration
The mean self-reported duration of total sleep time across the entire sample was 6.8 ± 1.2 h. Sleep duration was minimally different across all age groups: 45–54 years (6.7 ± 1.2 h), 55–64 years (6.8 ± 1.2 h), 65–74 years (6.9 ± 1.2 h), 75+ years (6.9 ± 1.3 h). The majority of participants reported sleeping greater than or equal to 6 h per night (n = 24,733, 86.8%).
Insomnia and insomnia symptoms
Across the entire sample, 13,385 (47.0%) of participants reported never experiencing sleep latency greater than 30 min in the past month, 10,588 (37.2%) reported sleep onset latency problems less than 3 times per week, while 4,450 (15.6%) experienced problems 3+ times per week. A third (33.2%, n = 9,452) of participants reported never experiencing problems maintaining sleep, 12,230 (42.9%) reported sleep maintenance problems less than twice per week, while 6,772 (23.8%) experienced problems 3+ times per week. There were more women than men in those experiencing difficulties getting to sleep (64.7% were females of those reporting 3+ times per week) or maintaining sleep (58.7% were females of those reporting 3+ times per week, Supplementary Table 1).
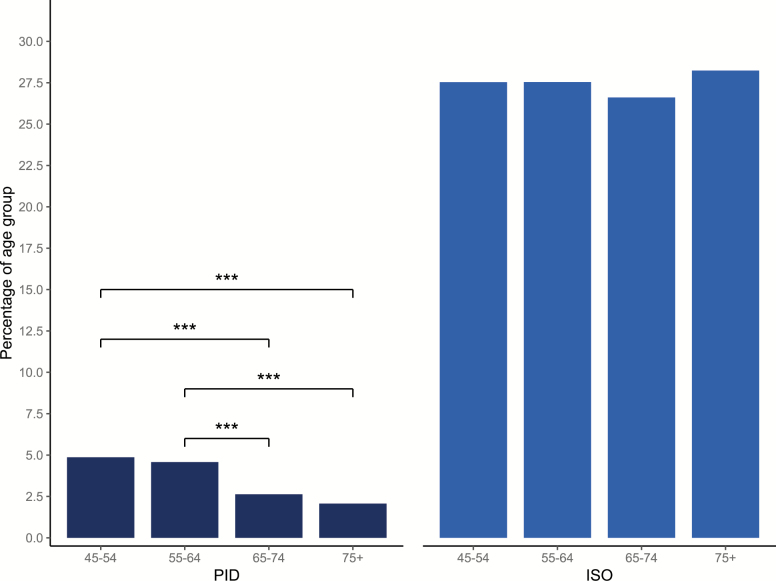
Of the entire sample, 1,068 participants (3.7%) met the criteria for PID. A further 7,813 participants (27.5%) reported ISO of delayed sleep onset latency or nocturnal awakenings more than 3 times per week, but did not report that this significantly impacted on their daily functioning. Most participants (19,604; 68.8%) were classified as presenting NIS. The prevalence of PID significantly decreased with age, with greater percentages of adults aged between 45–54 and 55–64 years reporting PID than adults aged 65–74 and 75+ (Figure 1). The prevalence of ISO did not differ across any age category.
Figure 1.
Percentage of adults reporting PID or ISO across age brackets. *p < 0.05; **p < 0.01; ***p < 0.001.
Table 2 displays the demographic, lifestyle, and medical characteristics for all groups. Almost every measure was significantly different between PID and the rest of the sample. Particularly, participants with PID had a greater prevalence of daytime sleepiness (65.9% vs. 37.0%), a greater BMI (29.0 vs. 27.9 kg m2) and were more likely to be smokers (12.1% vs. 6.6%). Additionally, PID participants exhibited a higher prevalence of affective disorders such as anxiety (24.8% vs. 7.9%) and depression (38.3% vs. 15.3%). Participants with ISO also exhibited significant demographic and lifestyle differences from those with NIS (Table 2).
Table 2.
Demographic, clinical, and lifestyle characteristics of participants with PID, ISO, and NIS
| PID | ISO | NIS | PID vs. ISO | PID vs. NIS | ISO vs. NIS | |
|---|---|---|---|---|---|---|
| (n = 1,068) | (n = 7,813) | (n = 19,604) | P | P | P | |
| Age, years ± SD | 59.75 ± 9.15 | 62.82 ± 10.27 | 62.93 ± 10.22 | <0.001 | <0.001 | 0.747 |
| Females, % | 64.5 | 58.3 | 48.2 | <0.001 | <0.001 | <0.001 |
| Primary language | ||||||
| English | 80.5 | 79.7 | 78.0 | 0.555 | 0.059 | 0.003 |
| French | 16.1 | 18.1 | 19.4 | 0.135 | 0.011 | 0.015 |
| Caucasian, % | 93.6 | 96.1 | 95.5 | 0.001 | 0.007 | 0.033 |
| >12 years education, % | 82.7 | 84.0 | 85.8 | 0.002 | <0.001 | <0.001 |
| Income | ||||||
| <$20,000 | 12.1 | 6.1 | 4.1 | <0.001 | <0.001 | <0.001 |
| $20,000–$50,000 | 25.8 | 22.4 | 20.0 | 0.017 | <0.001 | <0.001 |
| $50,000–$100,000 | 30.4 | 32.2 | 33.5 | 0.277 | 0.049 | 0.049 |
| $100,000–$150,000 | 15.0 | 17.7 | 19.2 | 0.033 | 0.001 | 0.008 |
| $150,000+ | 10.4 | 15.1 | 16.9 | <0.001 | <0.001 | <0.001 |
| BMI, kg m2 ± SD | 28.99 ± 6.1 | 27.93 ± 5.34 | 27.88 ± 5.08 | <0.001 | <0.001 | 0.747 |
| Smoker, % | 12.1 | 7.4 | 6.2 | <0.001 | <0.001 | 0.030 |
| Alcohol >4 times per week, % | 17.4 | 26.8 | 26.3 | <0.001 | <0.001 | 0.016 |
| Anxiety disorder, % | 24.8 | 9.7 | 7.1 | <0.001 | <0.001 | <0.001 |
| Current or history of Depression,% | 38.3 | 18.2 | 14.0 | <0.001 | <0.001 | 0.816 |
| Taking antidepressant medication | 22.9 | 7.9 | 7.1 | <0.001 | <0.001 | <0.001 |
| CESD score ± SD | 11.1 ± 6.2 | 6.6 ± 4.8 | 4.3 ± 4.1 | <0.001 | <0.001 | <0.001 |
| Breathing stops in sleep, % | 24.6 | 13.4 | 13.5 | <0.001 | <0.001 | 0.839 |
| Daytime sleepiness % | 65. 9 | 41.7 | 34.9 | <0.001 | <0.001 | <0.001 |
| Chronic pain, % | 59.1 | 40.7 | 31.2 | <0.001 | <0.001 | <0.001 |
| Cancer, % | 14.5 | 16.1 | 14.9 | 0.206 | 0.747 | 0.017 |
| Diabetes mellitus, % | 14.0 | 9.9 | 8.4 | <0.001 | <0.001 | <0.001 |
| Systolic BP, mmHg2 | 120.4 ± 16.3 | 121.1 ± 16.7 | 120.9 ± 16.6 | 0.631 | 0.370 | 0.660 |
| Diastolic BP, mmHg2 | 74.8 ± 10.4 | 73.9 ± 9.9 | 73.7 ± 10.0 | 0.001 | 0.012 | 0.181 |
CESD = Center for Epidemiologic Studies Depression Scale; BMI = body mass index; BP = blood pressure. Tukey’s honest significance test or χ2 test, as appropriate. All analyses are FDR adjusted to correct for multiple comparisons.
Bold and italicized text refers to values that pass the FDR adjusted threshold for statistical significance.
Associations between insomnia and cognitive function
The estimated marginal means for neuropsychological test scores in each group for model 1 and model 2 are reported in Table 3. Raw distributions of scores on all tests in each group are presented in Supplementary Figure 3, and effect sizes for each group level comparison on all tests are reported in Supplementary Table 2. After adjusting for age, sex, and education (model 1), the PID group performed significantly worse compared with participants with NIS or ISO on every cognitive test except for the COWAT and CRT.
Table 3.
Cognitive performance scores between subjects with PID, ISO, and NIS (adjusted means [95% confidence intervals])
| Cognitive test | PID | ISO | NIS | PID vs. ISO | PID vs. NIS | ISO vs. NIS | |
|---|---|---|---|---|---|---|---|
| (n = 1,068) | (n = 7,813) | (n = 19,604) | P | P | P | ||
| Processing speed | |||||||
| Stroop 1, s | Model 1 | 13.08 (12.90–13.27) | 12.83 (12.75–12.92) | 12.89 (12.82–12.95) | 0.032 | 0.056 | 0.190 |
| Model 2 | 12.33 (11.18–13.47) | 12.41 (11.28–13.53) | 12.43 (11.31–13.56) | 0.617 | 0.617 | 0.617 | |
| Stroop 2, s | Model 1 | 17.42 (17.16–17.68) | 16.99 (16.87–17.11) | 16.98 (16.89–17.07) | 0.003 | 0.003 | 0.844 |
| Model 2 | 17.29 (15.66–18.92) | 17.37 (15.77–18.97) | 17.41 (15.81–19.01) | 0.658 | 0.658 | 0.658 | |
| Executive functions | |||||||
| Stroop 3, s | Model 1 | 28.93 (28.35–29.50) | 27.98 (27.73–28.24) | 28.09 (27.89–28.30) | 0.005 | 0.007 | 0.371 |
| Model 2 | 30.88 (27.05–34.72) | 30.42 (26.65–34.18) | 30.47 (26.71–34.24) | 0.658 | 0.658 | 0.658 | |
| COWAT, words | Model 1 | 36.62 (35.85–37.40) | 36.70 (36.36–37.04) | 36.94 (36.66–37.21) | 0.850 | 0.639 | 0.471 |
| Model 2 | 38.55 (33.52–43.57) | 38.03 (33.09–42.96) | 38.13 (33.20–43.06) | 0.649 | 0.649 | 0.649 | |
| AFT, words | Model 1 | 19.20 (18.85–19.55) | 19.62 (19.47–19.78) | 19.70 (19.57–19.82) | 0.033 | 0.016 | 0.323 |
| Model 2 | 19.13 (16.87–21.39) | 19.25 (17.03–21.48) | 19.33 (17.11–21.55) | 0.632 | 0.632 | 0.632 | |
| MAT, items | Model 1 | 23.94 (23.41–24.46) | 25.37 (25.14–25.61) | 25.20 (25.01–25.39) | <0.001 | <0.001 | 0.124 |
| Model 2 | 24.34 (20.95–27.74) | 25.23 (21.90–28.57) | 24.93 (21.60–28.26) | 0.020 | 0.117 | 0.041 | |
| Memory | |||||||
| RAVLT I, words | Model 1 | 5.43 (5.32–5.54) | 5.58 (5.53–5.63) | 5.58 (5.55–5.62) | 0.015 | 0.015 | 0.797 |
| Model 2 | 5.22 (4.53–5.92) | 5.37 (4.69–6.06) | 5.33 (4.65–6.02) | 0.177 | 0.204 | 0.204 | |
| RAVLT II, words | Model 1 | 3.51 (3.39–3.64) | 3.71 (3.65–3.76) | 3.73 (3.68–3.77) | 0.004 | 0.002 | 0.519 |
| Model 2 | 3.18 (2.38–3.97) | 3.42 (2.64–4.20) | 3.41 (2.63–4.19) | 0.010 | 0.010 | 0.905 | |
| Reaction time | |||||||
| CRT, s | Model 1 | 837.76 (825.32–850.20) | 824.83 (819.32–830.33) | 827.65 (823.29–832.02) | 0.145 | 0.166 | 0.292 |
| Model 2 | 850.36 (768.35–932.37) | 844.45 (763.82–925.08) | 847.19 (766.69–927.68) | 0.999 | 0.999 | 0.998 |
Model 1 = adjusted for age, sex, income, years of education; model 2 = adjusted for age, sex, income, years of education, body mass index, snoring, daytime sleepiness, frequency of alcohol intake, diagnosis of cancer, anxiety, depression, chronic pain; COWAT = Controlled Oral Word Association Test; AFT = Animal Fluency Test; MAT = Mental Alternation Test; RAVLT I = Rey Auditory Verbal Learning Test—Encoding Phase; RAVLT II = Rey Auditory Verbal Learning Test—Recall Phase; CRT = choice reaction time. All analyses are FDR adjusted to correct for multiple comparisons.
Bold and italicized text refers to values that pass the FDR adjusted threshold for statistical significance.
After adjusting for other comorbidities and lifestyle factors (model 2) the relationship between scores of PID and NIS groups were no longer significant, except for the memory recall phase of the RAVLT (3.18 vs. 3.41 words, p = 0.010 FDR corrected). Participants with PID also performed worse than those with ISO on the memory recall phase of the RAVLT (3.18 vs. 3.42 words, p = 0.010 FDR corrected). Performances on the MAT became significantly greater in those with ISO compared with the NIS group (25.23 vs. 24.93 items, p = 0.041 FDR corrected).
After further adjustment for whether participants had an average sleep duration of ±6 h (model 3), all relationships from model 2 remained. Participants with PID still had significantly worse scores than the NIS group on the memory recall phase of the RAVLT (3.17 vs 3.40 words, p = 0.030 FDR adjusted). The PID group also still performed worse than those with ISO on the memory recall phase of the RAVLT (3.17 vs 3.37 words, p = 0.036 FDR adjusted). The ISO group still performed significantly better on the MAT after adjusting for average sleep duration compared with the NIS group (25.14 vs. 24.74, p = 0.035 FDR adjusted) and PID group (25.14 vs. 24.30 items, p = 0.047 FDR adjusted). Differences in estimated marginal means for each group split by self-reported sleep time less than and greater than or equal to 6 h are reported in Supplementary Table 3.
Sleep onset insomnia versus sleep maintenance insomnia
Based upon the types of insomnia symptoms they reported, 63 (5.9%) PID participants reported only sleep onset symptoms, 202 (18.9%) experienced only sleep maintenance symptoms, and the majority (78.2%) reported a mix of both. Females were more likely to experience sleep onset symptoms (69.8% of those reporting sleep onset symptoms) while maintenance symptoms were more equally reported across both females and males (53% vs. 47% of those reporting sleep maintenance symptoms). Additionally, there were no significant differences in performances on any cognitive test between participants with PID who reported sleep onset or sleep maintenance symptoms (Supplementary Table 4).
Decision tree regression of cognitive function in older adults with PID
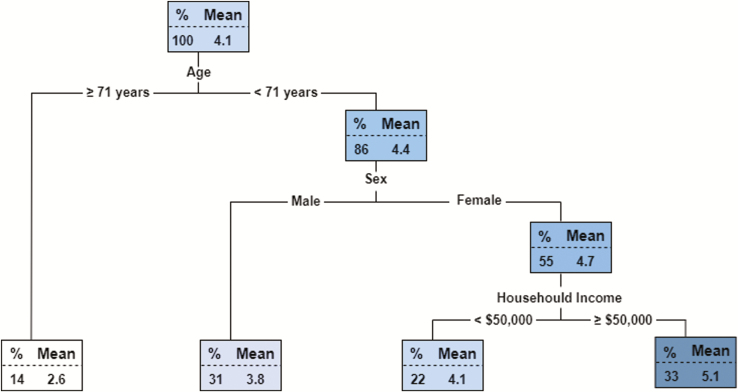
As performance on the memory component of the RAVLT was the only outcome to survive multivariate adjustment, a decision tree regression model was used to determine the most discriminant variables included in the multivariate model (model 3) that predicted overall performance on this test in PID (Figure 2). The single best predictor of high performance was age <71 years. The other variables determined to be most important for higher memory recall performance in PID included: females, and having a household income of greater than or equal to $50,000.
Figure 2.
Decision tree regression model for performance on the delayed recall phase of the RAVLT in PID. Each node of the tree reflects the mean score on the test and percentage of the sample for each group split by the preceding variable.
Discussion
Utilizing data from a large cohort of cognitively healthy middle-aged and elderly Canadian adults, the aim of this study was to assess the independent association between insomnia disorder and cognitive function. Symptoms that intently reflected standardized DSM-V diagnostic criteria for insomnia [3] were used to identify PID, while participants who reported insomnia symptoms without complaints of daytime dysfunction were classified as exhibiting ISO. This allowed not only for the examination of cognitive performance associated with insomnia disorder in a large sample of adults, but also to differentiate between the effects of insomnia symptoms and insomnia as a disorder defined by complete clinical diagnostic criteria.
Following adjustment for age, sex, education, and household income, adults with PID demonstrated small yet statistically significant impairments across a range of neuropsychological measures of cognitive function than adults with either ISO or NIS. This is presumably influenced by the clinical characteristics of subjects with PID, as these participants exhibited greater proportions of anxiety, depression, diabetes, smoking, daytime sleepiness, and breathing stoppages during sleep (an indirect gauge of obstructive sleep apnea), in addition to greater BMI and diastolic blood pressure—all associated with cognitive impairment [37–42] and regarded as risk factors for cognitive decline [43]. After adjustment of these factors, regression models revealed that only performance on a test of declarative memory remained worse in participants with PID compared to participants with either ISO or NIS, thus indicating an association between insomnia disorder and memory function—independent from the other clinical characteristics of adults with insomnia disorder. Sleep has been repeatedly implicated in the consolidation of memory [44], including declarative memories [45, 46]. Additionally, worse subjective sleep quality is also associated with elevated amyloid pathology in the cerebrospinal fluid of older adults [47], while amyloid burden in the prefrontal cortex has been associated with poorer impaired overnight sleep-dependent memory retention [48]. A specific deficit in older adults with insomnia disorder is thus in line with previous work relating sleep to memory consolidation.
The reasons why these effects were specific to PID and not also to those with ISO is less clear. This is possibly reflective of the criteria used to define PID, for example the requirement of a subjective report of daytime impairment specifically related to each sleep complaint. While these reports were nonspecific to any particular domain, memory concerns are the most predominant cognitive complaint in older adults [49], suggesting memory complaints were common in the PID group. Thus, these subjective impairments that distinguished the PID and ISO groups may be detected in objective testing, and may partly explain why memory deficits were specifically reserved to the group with PID alone. Another important distinguishing factor between PID and ISO is the recurrence and chronicity of sleep disruption. It might be the case that cognitive disturbances take time to become manifest and that the persistence of insomnia over time—as seen in insomnia disorder—is required for deficits in memory consolidation to occur. Therefore, some degree of insomnia severity might be needed for cognitive deficits to be detected, in line with findings from other insomnia cohorts [23, 24].
While these effects are small, they are similar to effect sizes observed in other large scale epidemiological studies of insomnia symptoms [19]. This is likely due to the heterogeneity of insomnia disorder and insomnia symptoms across large populations, in addition to the wide range of additional contributing risk factors for cognitive impairment. A meta-analysis of previous smaller case–control studies of cognition in insomnia also found that moderate impairments existed in episodic memory compared to healthy controls [16]. This meta-analysis additionally observed significant impairments in problem solving and working memory, which was not replicated by the current findings, however it is likely that this is reflective of the contrast in methodological characteristics (e.g. inclusion criteria, population) between this study and the smaller clinical studies included in the meta-analysis. Two previous representative cross-sectional studies both failed to observe any cognitive impairments associated with PID [23, 24]. Within both studies, only certain insomnia subgroups exhibited poorer cognitive performances. In the first study of 1,037 individuals, only health-seeking insomnia subjects (n = 186) performed worse on tests of processing speed and verbal comprehension [23]. However, these subjects were significantly younger (all 38 years old) than the current sample, and most treatment seeking adults had experienced cognitive capacities below population norms since childhood. In the other study of 678 subjects, who were also on average younger (mean 50 years) than the current cohort, deficits were detected on measures of processing speed, mental flexibility, and short-term visual memory solely in insomnia subjects with objective short sleep duration (<6 h total sleep time) [24]. The current analyses demonstrated that deficits in memory performance was not statistically related to sleep duration in the group with PID. Regression tree analysis demonstrated that age was the single best predictor declarative memory performance in adults with PID. Specifically, participants with PID aged greater than 71 years showed the poorest memory performance. These regression analyses also identified that females exhibited less impairment than males. This is unsurprising, given the strong associations that age and sex share with memory and neuropsychological functioning generally [50, 51]. The regression analysis also indicated that a household income greater than $50,000 was also a discriminating variable. Although the interaction between insomnia and income level have not been associated with cognitive performance before, there is evidence that females with higher income demonstrate higher memory scores [52]. Overall, these findings suggest that females under the age of 71 with a mid-to-high income may be better protected from the negative outcomes in memory performance associated with insomnia disorder, and that other clinical or lifestyle factors do not appear to significantly further predict memory performance within insomnia disorder.
The identification of declarative memory impairment independently associated with insomnia in older adults is particularly important in the context of cognitive decline. Memory is one of the cognitive domains most affected in aging and is particularly impacted in certain dementias such as Alzheimer’s disease. While these current findings are limited to cross-sectional cognitive function in cognitively healthy middle-aged and older adults, the PID group also had significantly greater proportions of a range of adverse health outcomes that place them at an elevated risk for cognitive decline. For example, the PID group exhibited greater proportion of daytime sleepiness than the other groups. Evidence from clinical studies suggest that patients with insomnia do not often experience daytime sleepiness, however associations between excessive daytime sleepiness (EDS) and insomnia symptoms have previously been reported in older adults [53]. Therefore, older adults with insomnia may exhibit more daytime sleepiness, which has been independently associated with risk of cognitive decline [54, 55]. The assessment of longitudinal data within this cohort as it becomes available will be critical to understand if insomnia disorder may increase the risk of memory decline, or the presence of adverse risk factors for cognitive decline in aging.
Surprisingly, participants with ISO performed better than those with PID and NIS on the test of mental flexibility after adjusting for demographic, lifestyle, and medical factors, as well as subjective sleep duration. This is in contrast to a large body of literature that have implicated symptoms of sleep disturbance in cognitive impairment and increased risk of cognitive decline among older adults [56, 57]. However, a recent analysis of the UK Biobank reported similar findings of improved cognitive performance in adults with frequent insomnia symptoms, only after adjusting for demographic and medical comorbidities [19]. The authors interpreted this in the context of insomnia-related hyperarousal and personality traits, however the current findings suggest that these advantages are associated with ISO, not insomnia disorder. The distinction in performance between ISO and insomnia disorder (which required a report of daytime dysfunction) implies that older adults who are subjectively less sensitive to the effects of insomnia symptoms might also demonstrate slight objective resilience in cognitive performance. However, these effects appear only after statistical adjustment for clinical characteristics, demonstrating that some characteristics of adults with ISO act as negative confounding variables. Why insomnia symptoms, particularly without any subjective report of daytime impairments, might be associated with better performance in some cognitive domains needs to be further explored.
The prevalence of PID in this sample (3.7%) was measured to be less than half of what has previously been reported in the general population (9%–10%) [1, 2]. This may be explained by the features of the sample. Firstly, the CLSA only consists of adults older than 45 years of age. In a previous cross-sectional epidemiological study from a similarly sized sample of European adults only a third of elderly subjects with a predominant complaint of insomnia received a DSM-IV diagnosis of insomnia [2]. Indeed, the prevalence of insomnia symptoms in the current sample was much higher than PID, although still much lower than what has been previously reported [2, 58]. Furthermore, another study also using diagnostic criteria similar to the DSM-IV reported that the prevalence of insomnia disorder in Chinese adults aged more than 65 years was 8.9%—closely aligned with estimates in the general population [58]. While different estimates in insomnia prevalence may potentially arise from cultural, societal, or environmental characteristics of distinct samples, these would be unlikely to account for a two-fold increase in insomnia or insomnia symptoms. A major factor that may have influenced these differences could involve the CLSA study design of including at baseline only adults with an absence of cognitive impairment, dementia, or history of brain injury. Given that sleep disturbances in later life are strongly associated with cognitive decline and dementia, and insomnia often occurs following brain injury [59], it is plausible that much higher rates of insomnia and insomnia symptoms are present in these adults who were excluded from the current study, explaining the much lower observed prevalence of insomnia and insomnia symptoms than those from studies of the general population. The decline in insomnia disorder with age (and stability of insomnia symptom prevalence) may therefore reflect a healthy survivor-effect. Particularly, insomnia occurring less frequently in successful aging further supports a link between sleep disorders and cognitive decline, as older adults successfully aging without cognitive impairment are less likely to have insomnia disorder. This could be supported by longitudinal data detailing the precise relationship between insomnia disorder and cognitive decline in later life.
The strengths of this study include the power achieved through such a large sample size, the inclusion of objective neuropsychological testing, and the phenotyping of insomnia disorder in comparison to ISO. Nevertheless, there are a few limitations that must be considered when interpreting the findings. Firstly, while insomnia disorder was measured using questions closely aligned to DSM-V criteria, these were not part of a standardized clinical interview conducted by a trained psychiatrist or sleep medicine specialist. For example, the question surrounding sleep satisfaction might have been interpreted in different ways by different participants (e.g. refreshing sleep vs. satisfaction with time of sleep onset or offset). Therefore, PID cannot be regarded as a definite in-person diagnosis of insomnia disorder, however such a diagnosis is unlikely to be achieved in such a large study. Secondly, while proxy measures of the presence of sleep apnea (daytime sleepiness and witnessed apneic events) were available and included in the models, objective information surrounding any sleep disorders, or the use of sleeping medication, were not available and thus could not be incorporated into these analyses. These data come from a specific study on healthy aging, and screening out cognitive impairment at baseline may have attenuated the associations between insomnia and cognition. As such these findings cannot be generalized to younger adults with insomnia under the age of 45, or older adults with moderate-to-severe objective cognitive impairments. Additionally, all the neuropsychological findings were of small magnitude. However, given that these data come from a cohort specifically chosen for the absence of any objective cognitive impairment, the fact that significant differences in memory performance were still able to be detected demonstrates the sensitive relationship between insomnia and memory in aging. This relationship may become stronger and more prevalent as cognitive function declines in the elderly. Finally, these findings are cross-sectional, and do not track the time course of cognitive function in insomnia in later life, which will need to be investigated to understand the distinct relationship between insomnia and cognitive decline. This highlights the benefit of longitudinal studies such as the CLSA to explore such factors for healthy aging.
In conclusion, these findings suggest that insomnia disorder in cognitively healthy middle aged and older adults is associated with deficits in a range of cognitive domains, which is likely due to the demographic and clinical characteristics of adults with insomnia. After adjustment for these variables, adults with PID exhibited decreased performance specifically on a test of declarative memory. This was distinguishable from ISO, and detectable despite the inclusion of adults without any identified cognitive impairment only. While small, these findings highlight the importance of distinguishing insomnia disorder from insomnia symptoms when considering cognitive function. The longitudinal relationship between insomnia disorder and memory decline now needs to be further explored in middle aged and older adults.
Supplementary Material
Acknowledgments
This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). This research has been conducted using the CLSA Baseline Comprehensive Dataset version 4.0, under Application Number 160607. The CLSA is led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland.
Funding
Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 9447 and the Canada Foundation for Innovation. N.C. is funded by the Fonds de Recherche du Québec (Santé) and the Canadian Sleep and Circadian Network. R.P. reports grants and personal fees from Fonds de la Recherche en Sante, the Canadian Institute of Health Research, Parkinson Canada, the Weston-Garfield Foundation, the Michael J. Fox Foundation, the Webster Foundation, and personal fees from Takeda, Roche/Prothena, Teva Neurosciences, Novartis Canada, Biogen, Boehringer Ingelheim, Theranexus, GE HealthCare, Jazz Pharmaceuticals, Abbvie, Jannsen, Phytopharmics, Inception Sciences, and Otsuko, outside the submitted work. N.G. is funded by a CIHR Foundation grant (#FDN154291) and FRQS Chercheur boursier (#34776). T.T.D.-V. is funded by the Natural Sciences and Engineering Research Council of Canada (RGPIN 436006-2013), the Canadian Institutes of Health Research (MOP 142191, PJT 153115, and PJT 156125), the Fonds de Recherche du Québec (Santé); the Canada Foundation for Innovation, and Concordia University.
Conflict of interest statement. None declared.
References
- 1. Morin CM, et al. . Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. [DOI] [PubMed] [Google Scholar]
- 2. Ohayon MM, et al. . Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the international classification of sleep disorders (ICSD). Sleep Med. 2009;10(9):952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 4. Jackson CL, et al. . Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asif N, et al. . Human immune system during sleep. Am J Clin Exp Immunol. 2017;6(6):92–96. [PMC free article] [PubMed] [Google Scholar]
- 6. Xie L, et al. . Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seow LSE, et al. . Evaluating DSM-5 insomnia disorder and the treatment of sleep problems in a psychiatric population. J Clin Sleep Med. 2018;14(2):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javaheri S, et al. . Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anothaisintawee T, et al. . Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. [DOI] [PubMed] [Google Scholar]
- 10. Benetó A, et al. . Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13(4):287–293. [DOI] [PubMed] [Google Scholar]
- 11. Altena E, et al. . Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17(3):335–343. [DOI] [PubMed] [Google Scholar]
- 12. Crenshaw MC, et al. . Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66(3):485–492. [DOI] [PubMed] [Google Scholar]
- 13. Haimov I, et al. . Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. [DOI] [PubMed] [Google Scholar]
- 14. Orff HJ, et al. . Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30(9):1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varkevisser M, et al. . Chronic insomnia and daytime functioning: an ambulatory assessment. Behav Sleep Med. 2007;5(4):279–296. [DOI] [PubMed] [Google Scholar]
- 16. Fortier-Brochu E, et al. . Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. [DOI] [PubMed] [Google Scholar]
- 17. Cricco M, et al. . The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49(9):1185–1189. [DOI] [PubMed] [Google Scholar]
- 18. Nebes RD, et al. . Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64(2):180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kyle SD, et al. . Sleep and cognitive performance: cross-sectional associations in the UK biobank. Sleep Med. 2017;38:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gildner TE, et al. . Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: results from the Study on Global Ageing and Adult Health (SAGE). J Clin Sleep Med. 2014;10(6):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johar H, et al. . Impaired sleep predicts cognitive decline in old people: findings from the prospective KORA age study. Sleep. 2016;39(1):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devore EE, et al. . Sleep duration in midlife and later life in relation to cognition. J Am Geriatr Soc. 2014;62(6):1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldman-Mellor S, et al. . Is insomnia associated with deficits in neuropsychological functioning? Evidence from a population-based study. Sleep. 2015;38(4):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandez-Mendoza J, et al. . Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raina PS, et al. . The Canadian Longitudinal Study on Aging (CLSA). Can J Aging. 2009;28(3):221–229. [DOI] [PubMed] [Google Scholar]
- 26. Bastien CH, et al. . Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 27. Buysse DJ, et al. . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 28. Ikehara S, et al. ; JACC Study Group Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32(3):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lezak M. Neuropsychological Assessment. New York: Oxford University Press; 1982. [Google Scholar]
- 30. Himmelfarb S, et al. . Reliability and validity of five mental health scales in older persons. J Gerontol. 1983;38(3): 333–339. [DOI] [PubMed] [Google Scholar]
- 31. Golden CJ. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Stoeling; 1978. [Google Scholar]
- 32. Spreen O, Benton AL.. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA). Victoria, AB: University of Victoria Neuropsychology Laboratory; 1977. [Google Scholar]
- 33. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013. http://www.r-project.org/. Accessed December 6, 2018. [Google Scholar]
- 34. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995; 57 (1): 289–300. [Google Scholar]
- 35. Therneau TM, et al. . An Introduction to Recursive Partitioning Using the RPART Routines. 2018. https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf Accessed August 27, 2018. [Google Scholar]
- 36. Breiman L, Friedman JH, Olshen RA, Stone CJ.. Classification and Regression Trees. Belmont, CA: Wadsworth; 1983. [Google Scholar]
- 37. Castaneda AE, et al. . A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1–2):1–27. [DOI] [PubMed] [Google Scholar]
- 38. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campos MW, et al. . Smoking and cognition. Curr Drug Abuse Rev. 2016;9(2):76–79. [DOI] [PubMed] [Google Scholar]
- 40. Fulda S, et al. . Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5(6):423–445. [DOI] [PubMed] [Google Scholar]
- 41. Bucks RS, et al. . Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18(1):61–70. [DOI] [PubMed] [Google Scholar]
- 42. Cournot M, et al. . Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208–1214. [DOI] [PubMed] [Google Scholar]
- 43. Norton S, et al. . Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. [DOI] [PubMed] [Google Scholar]
- 44. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013; 93 (2): 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu P, et al. . Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17(10):891–898. [DOI] [PubMed] [Google Scholar]
- 46. Plihal W, et al. . Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9(4):534–547. [DOI] [PubMed] [Google Scholar]
- 47. Sprecher KE, et al. . Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89(5):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mander BA, et al. . β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rabin LA, et al. . Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis. 2015;48(Suppl. 1):S63–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fjell AM, et al. . Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–221. [DOI] [PubMed] [Google Scholar]
- 51. Strauss EH, Sherman EMS, Spreen OA.. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 52. Lee S, et al. . The relation of education and income to cognitive function among professional women. Neuroepidemiology. 2006;26(2):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jaussent I, et al. . Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34(8):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elwood PC, et al. . Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health. 2011;65(9):820–824. [DOI] [PubMed] [Google Scholar]
- 55. Foley D, et al. . Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49(12):1628–1632. [DOI] [PubMed] [Google Scholar]
- 56. Spira AP, et al. . Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27(6):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yaffe K, et al. . Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. [DOI] [PubMed] [Google Scholar]
- 58. Liu X, et al. . Sleep habits and insomnia in a sample of elderly persons in China. Sleep. 2005;28(12):1579–1587. [PubMed] [Google Scholar]
- 59. Viola-Saltzman M, et al. . Traumatic brain injury-induced sleep disorders. Neuropsychiatr Dis Treat. 2016;12:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.