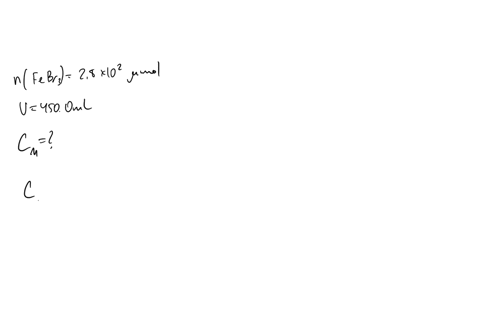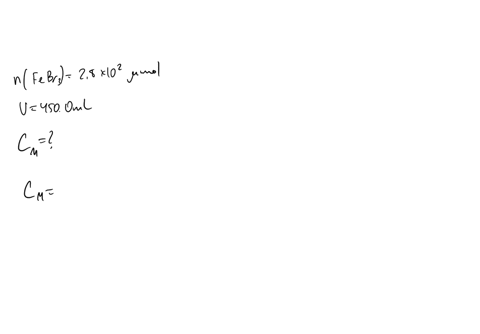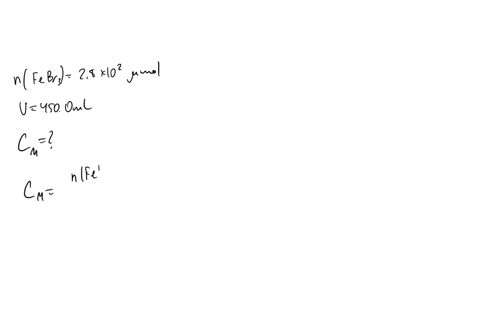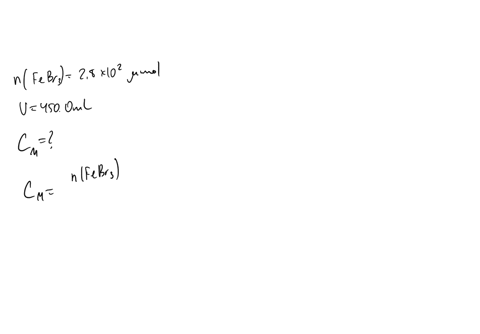Question
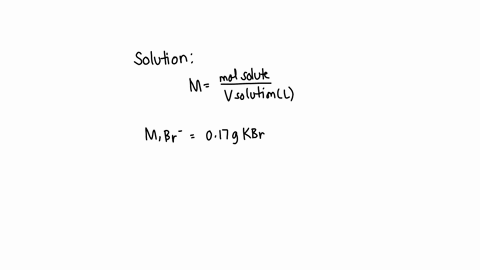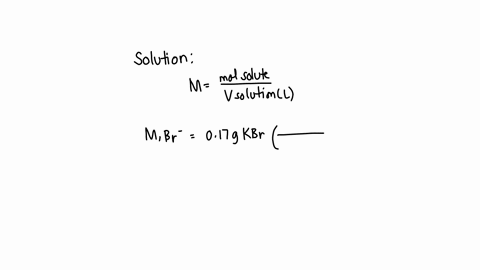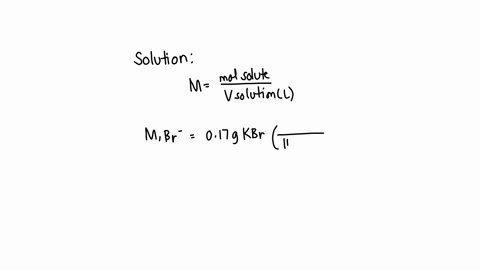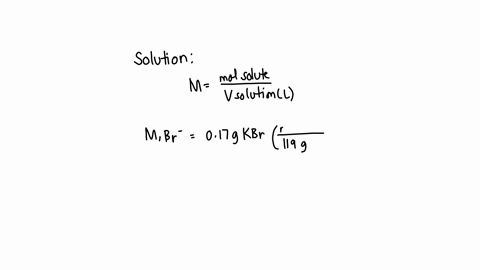
A chemist prepares a solution of sodium bromide (NaBr) by measuring out 0.60 g of NaBr into a 300 mL volumetric flask and filling it to the mark with distilled water. Calculate the molarity of Br- anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.
A chemist prepares a solution of sodium bromide (NaBr) by measuring out 0.60 g of NaBr into a 300 mL volumetric flask and filling it to the mark with distilled water. Calculate the molarity of Br- anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.
Show more…
Added by Cathy M.
Instant Answer
Step 1
To calculate the molarity of Br? anions in the chemist's solution, we need to know the concentration of Br? anions and the volume of the solution. Show more…
Show all steps