Question
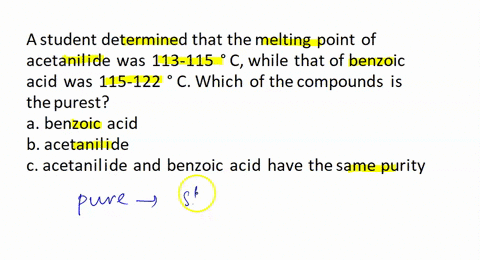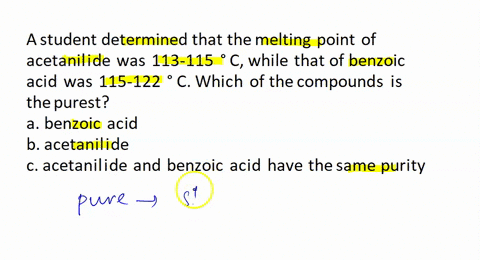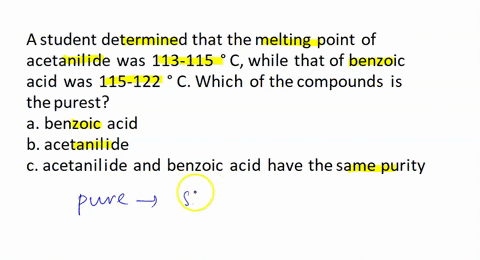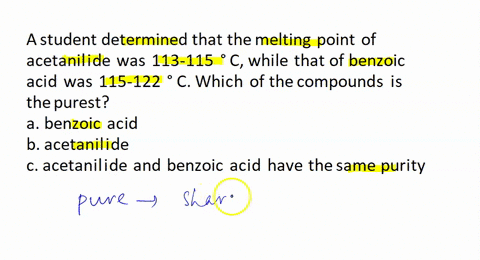
A student recrystallized some impure benzoic acid and isolated it by filtration. He scraped the purified benzoic acid off the filter paper after it had dried and took the melting point as a test for purity. He was surprised that most of the white solid melted sharply between 121 and 122 °C but that a small amount remained unmelted even at temperatures above 200 °C. Explain this behavior.
A student recrystallized some impure benzoic acid and isolated it by filtration. He scraped the purified benzoic acid off the filter paper after it had dried and took the melting point as a test for purity. He was surprised that most of the white solid melted sharply between 121 and 122 °C but that a small amount remained unmelted even at temperatures above 200 °C. Explain this behavior.
Show more…
Added by Mohamed B.
Instant Answer
Step 1
** Show more…
Show all steps