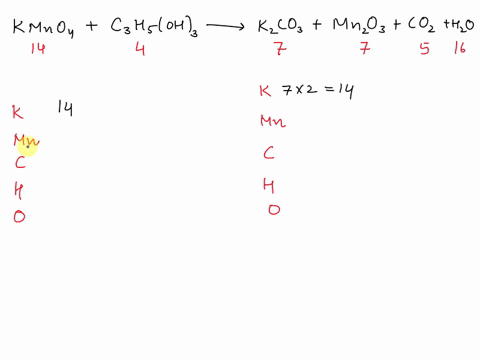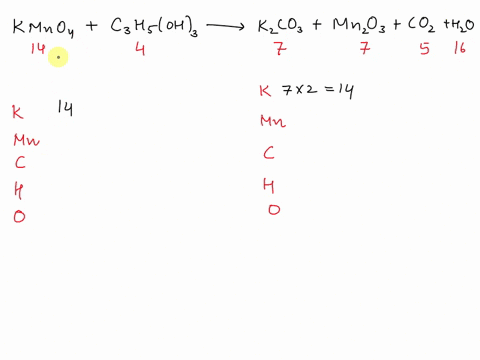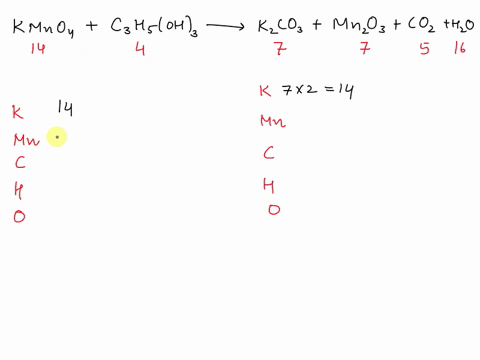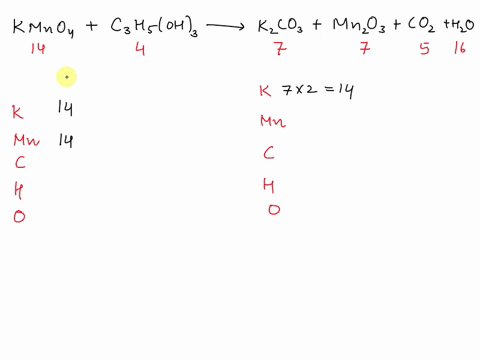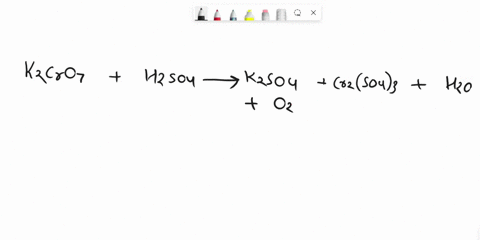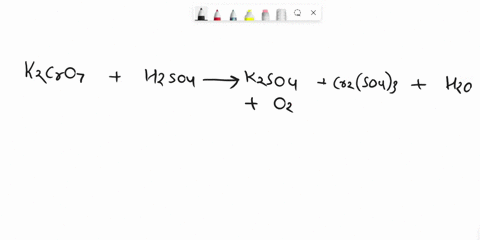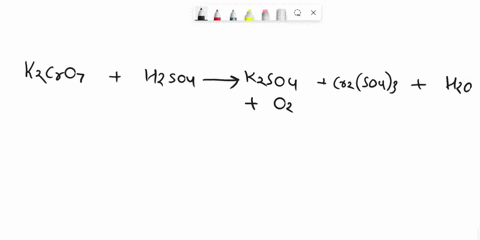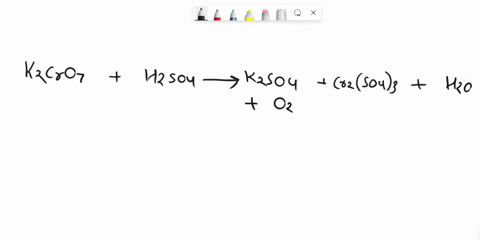Question
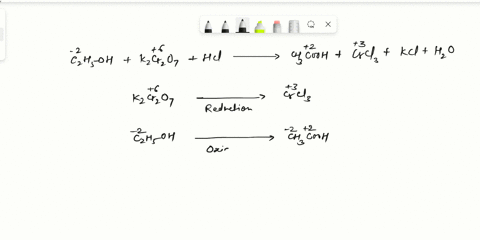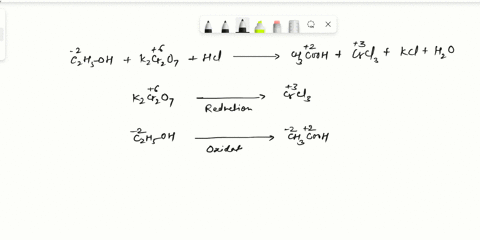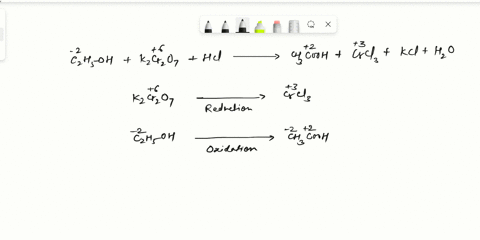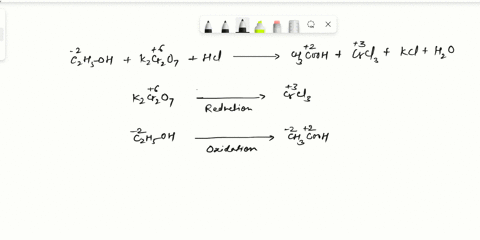
Balance Equation: K2Cr2O7 + H2C2O4 + 2H2O -> 6K[Cr(C2O4)2(H2O)2] + CO2 + H2O
Balance Equation:
K2Cr2O7 + H2C2O4 + 2H2O -> 6K[Cr(C2O4)2(H2O)2] + CO2 + H2O
Added by John B.
Instant Answer
Step 1
Starting with potassium (K), we have 2 on the left side and 6 on the right side, so we need to add a coefficient of 3 in front of K2Cr2O7: 3 K2Cr2O7 + H2C2O4 2H2O → 6 K[Cr(C2O4)2(H2O)2]2H2O + CO2 + H2O Show more…
Show all steps