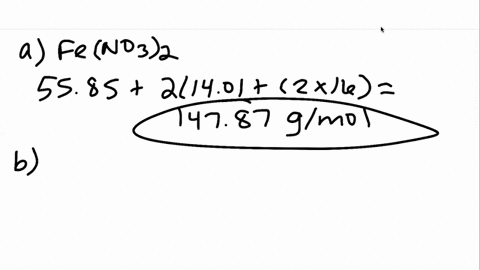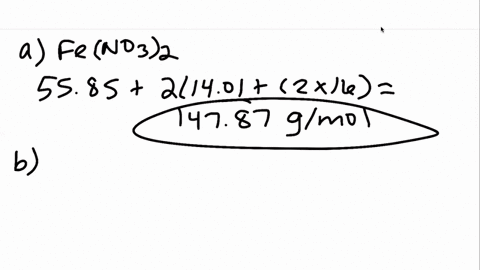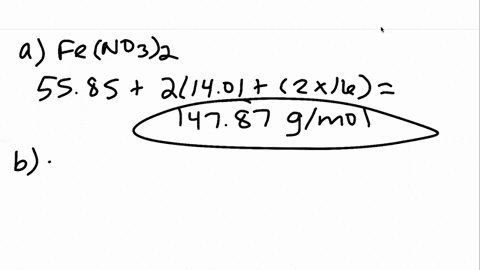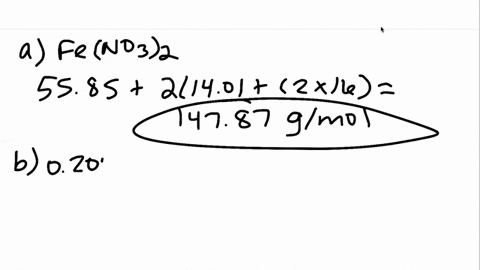
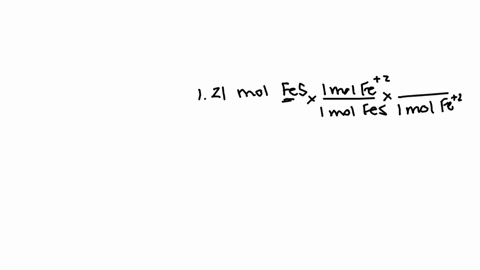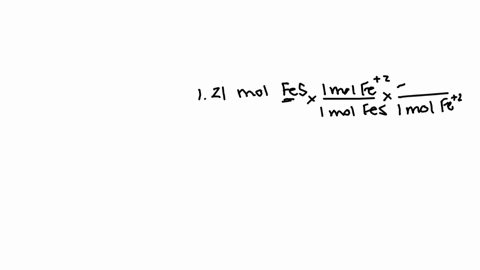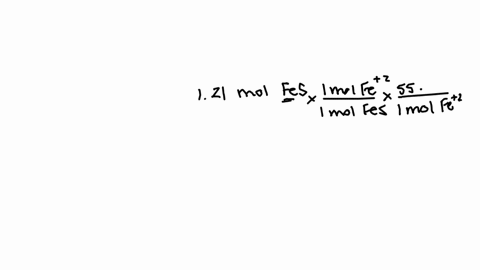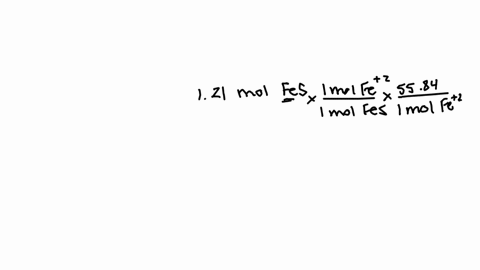00:01
Okay, so in order to determine the chemical formula for iron three sulfide, so you've got iron, which is f -e, and since it's three, you know that it has a positive three charge.
00:13
S, and we know that it's a monoatomic ion because the word is sulfide and not sulfite or sulfate, so we know it's not polyatomic.
00:22
So sulfur, we know from the periodic table, has a negative two charge.
00:27
So we want these charges to equal one another.
00:30
So we've got a positive three and we've got a negative two.
00:34
So positive three plus a negative two does not equal zero.
00:40
And we have to get these to equal zero in our formula in order to have the correct formula.
00:44
So we're going to do that, i've got to have three positive three charges.
00:47
Oh, sorry? i've got to have two positive three charges and three negative two charges.
00:52
This gives me a positive six plus a negative six.
00:57
An overall charge of zero.
00:59
So i've got to have three negative charges means i've got to have three sulfurs and i've got to have two fe3 plus ions.
01:08
So that means my formula then, if i wrote this out, two fe3 plus and three sulfur ions would give me a formula of fe2.
01:24
Oh, sorry, three.
01:30
So there's my chemical formula.
01:32
How many atoms are in one formula unit of this? that means i've got two iron atoms...