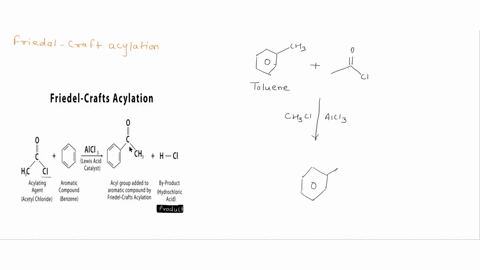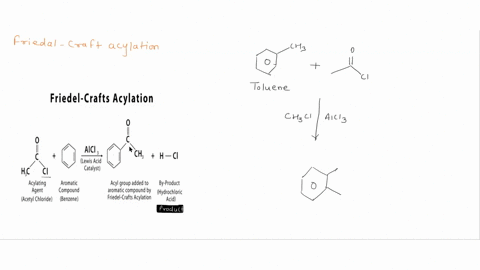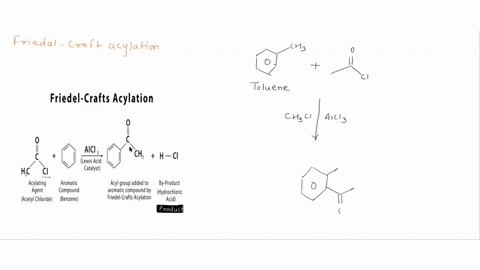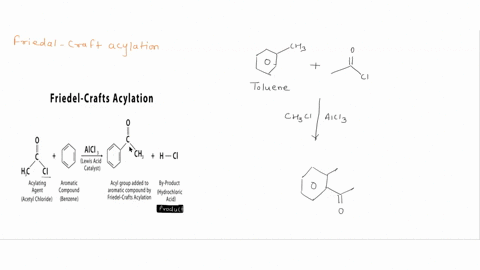
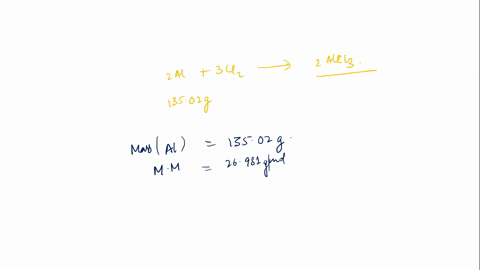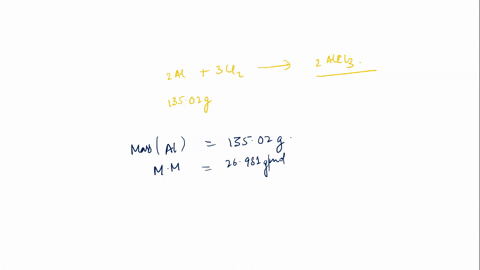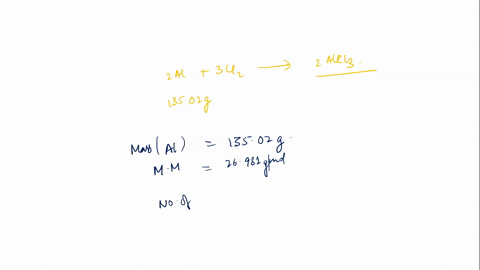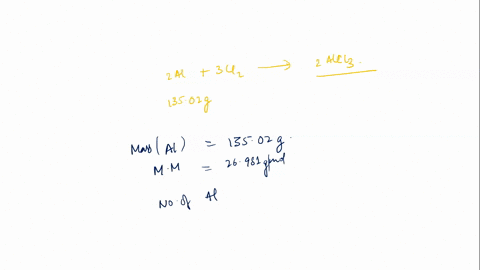Question
How do you calculate the theoretical yield of a polymer? If .02 moles of styrene are mixed with .05 moles of AlCl3, how many grams of polystyrene will be produced?
How do you calculate the theoretical yield of a polymer? If .02
moles of styrene are mixed with .05 moles of AlCl3, how many grams
of polystyrene will be produced?
Added by Michelle R.
Instant Answer
Step 1
In this scenario, styrene (C8H8) undergoes polymerization in the presence of a catalyst (AlCl3) to form polystyrene. The general reaction for polymerization of styrene can be represented as: \[ n(C_8H_8) \rightarrow (C_8H_8)_n \] This means that n moles of styrene Show more…
Show all steps