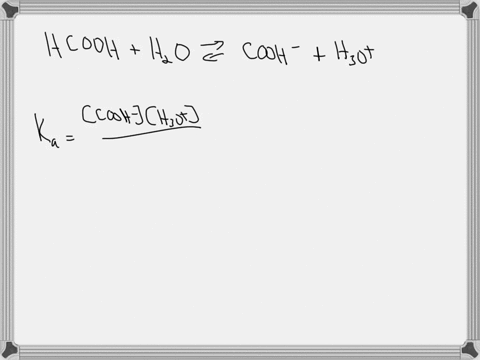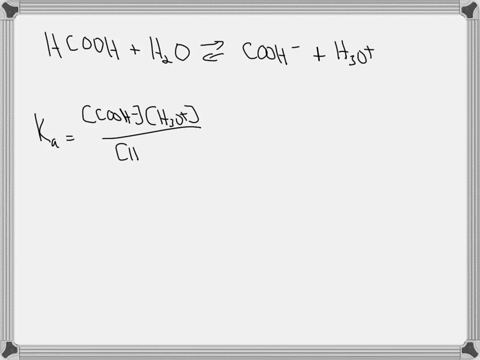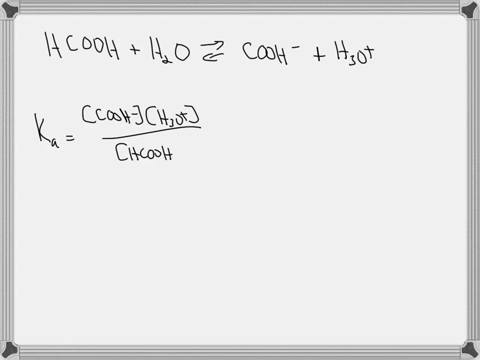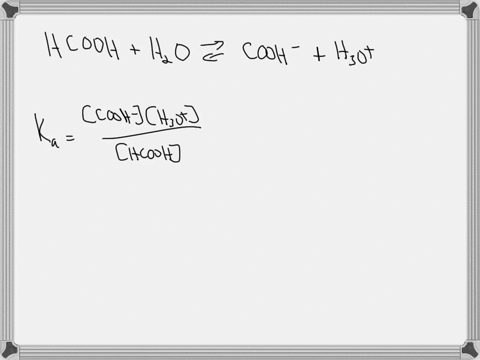Question
Hypoiodous acid (HIO) is a weak acid that dissociates in water as follows: HIO(aq) + H2O(l) → H3O+(aq) + IO−(aq). A 0.12 M solution of hypoiodous acid has a pH of 5.71. Calculate the acid-dissociation constant (Ka) for this acid.
Hypoiodous acid (HIO) is a weak acid that dissociates in water as follows:
HIO(aq) + H2O(l) → H3O+(aq) + IO−(aq).
A 0.12 M solution of hypoiodous acid has a pH of 5.71. Calculate the acid-dissociation constant (Ka) for this acid.
Show more…
Added by Clara R.
Instant Answer
Step 1
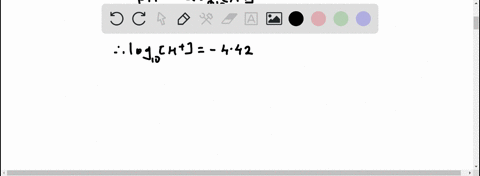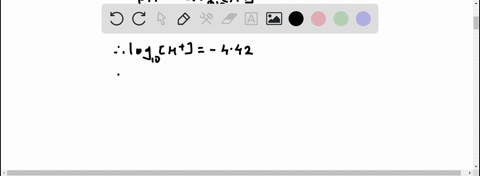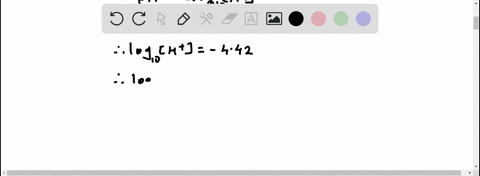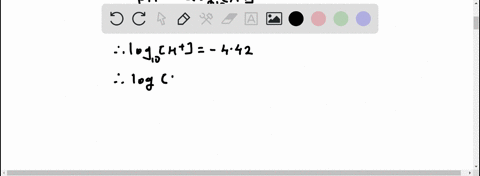
Given: pH = 5.71 [H3O+] = 10^(-pH) = 10^(-5.71) = 1.95 x 10^(-6) M [IO-] = [H3O+] = 1.95 x 10^(-6) M Show more…
Show all steps