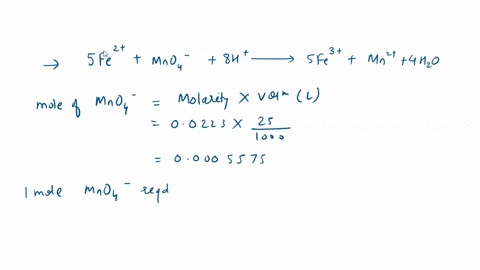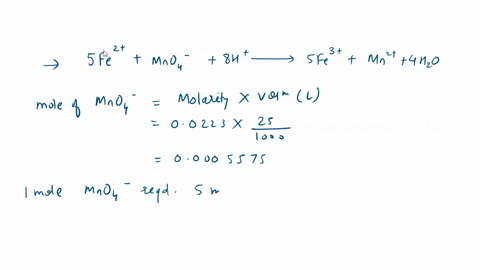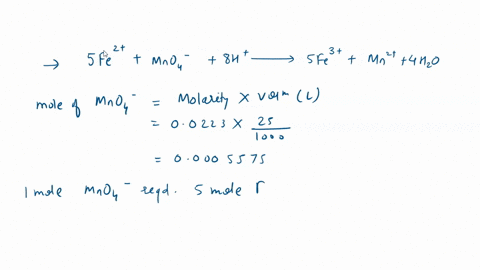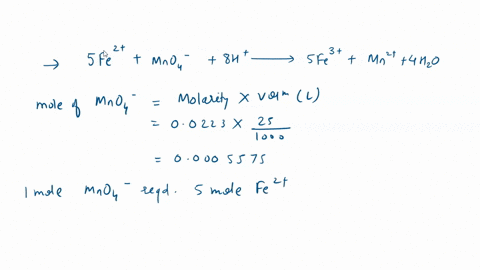Question
In a titration with potassium permanganate, iron(Il) chloride turns into iron(III) chloride: 1. Is iron oxidized or reduced in the reaction? Briefly explain; 2. Give the formula for potassium permanganate. Edit View Insert Format Tools Table 12pt Paragraph B I 4 4 ~ Ev T2 ~
In a titration with potassium permanganate, iron(Il) chloride turns into iron(III) chloride:
1. Is iron oxidized or reduced in the reaction? Briefly explain; 2. Give the formula for potassium permanganate.
Edit View Insert Format Tools Table
12pt
Paragraph
B I 4 4 ~ Ev T2 ~
Show more…

Added by Alejandro S.
Instant Answer
Step 1
Iron is oxidized in the reaction. This is because it loses electrons and its oxidation state increases from +2 to +3. This is evident from the fact that iron(II) chloride is converted to iron(III) chloride. Show more…
Show all steps