Question
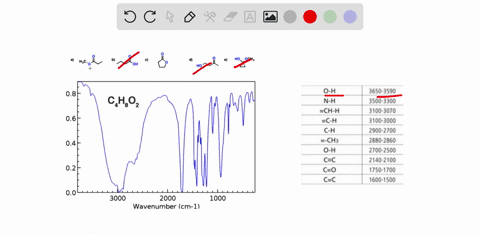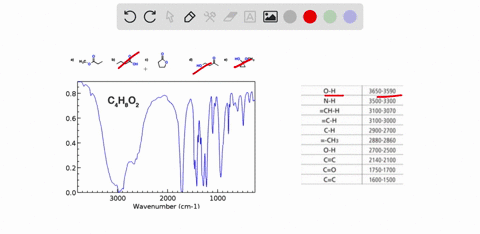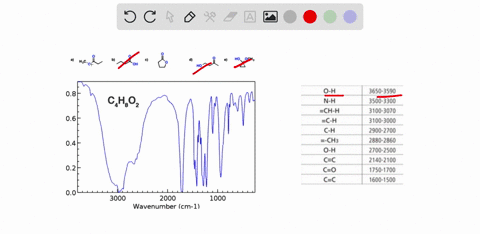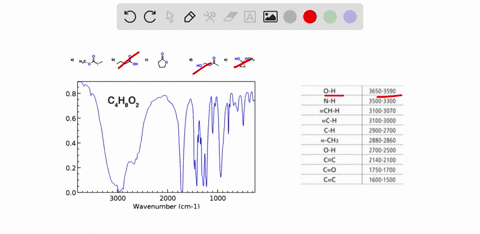
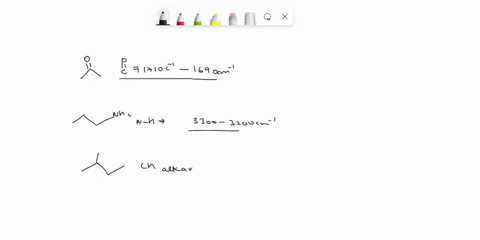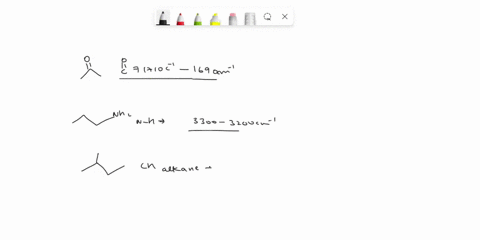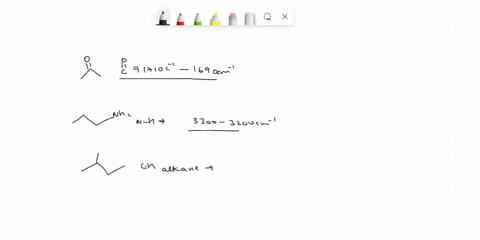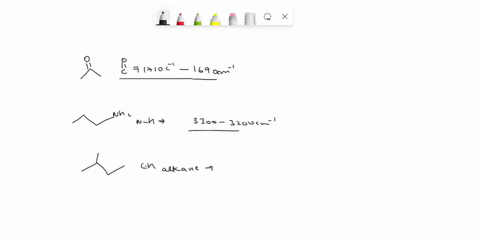
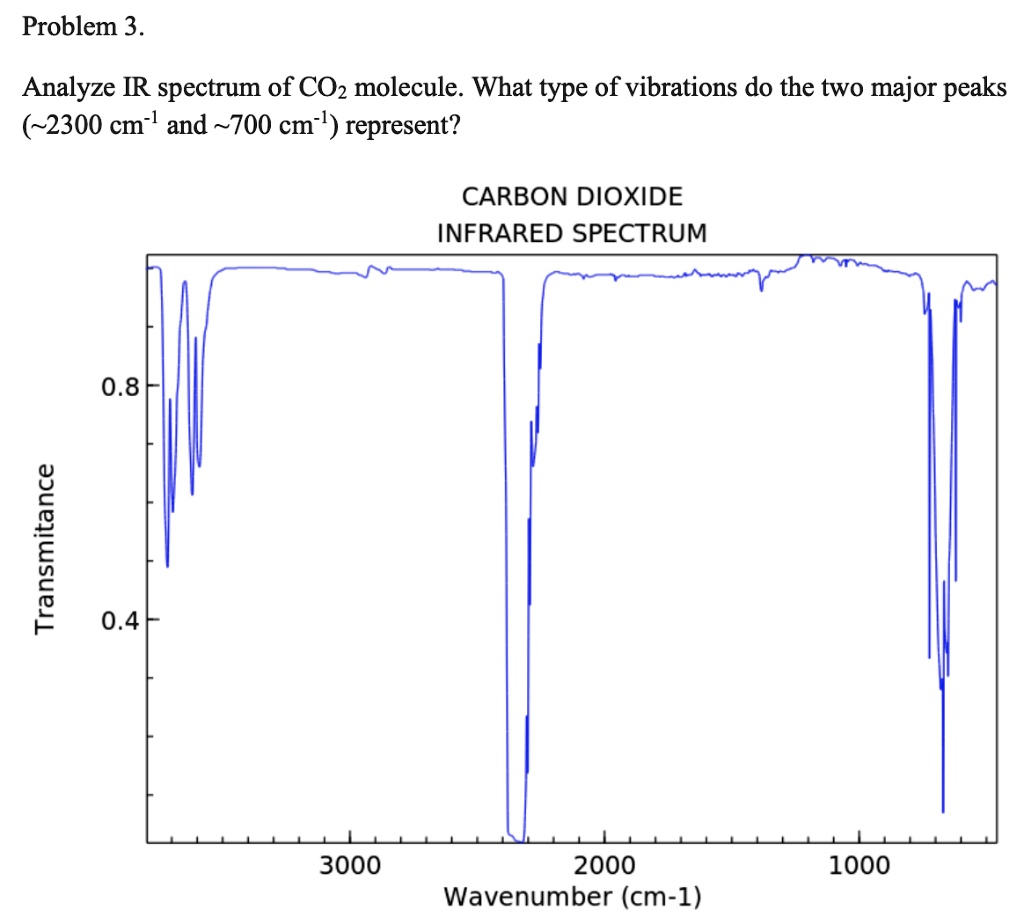
Problem 3 Analyze the IR spectrum of CO2 molecule. What type of vibrations do the two major peaks (~2300 cm-1 and ~700 cm-1) represent? CARBON DIOXIDE INFRARED SPECTRUM 0.8 L 0.4 3000 2000 1000 Wavenumber (cm-1)
Problem 3
Analyze the IR spectrum of CO2 molecule. What type of vibrations do the two major peaks (~2300 cm-1 and ~700 cm-1) represent?
CARBON DIOXIDE INFRARED SPECTRUM
0.8
L 0.4
3000
2000
1000
Wavenumber (cm-1)
Added by Carol K.
Instant Answer
Step 1
CO2 is a linear molecule with three atoms, so it has 3N - 5 = 3(3) - 5 = 4 vibrational modes. These modes can be classified as follows: Show more…
Show all steps