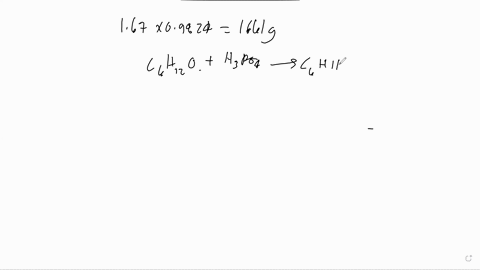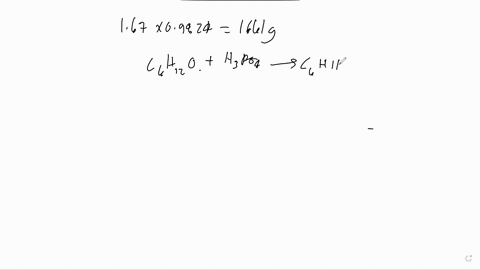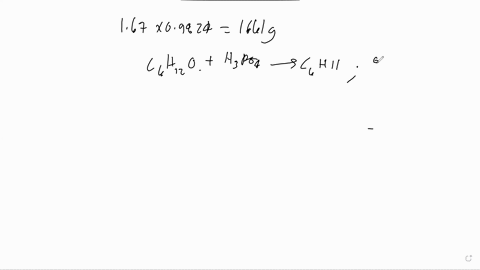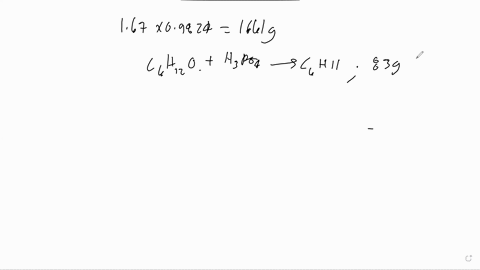Question
The following is the dehydration reaction that we would have performed in experiment 19. We would have converted cyclohexanol to cyclohexene. H3PO4 ILO Assume that we performed the above reaction starting with 5.7 grams of cyclohexanol. Calculate the theoretical yield of cyclohexene. Remember that the theoretical yield is the amount that you should get if the reaction goes 100% to completion. Show your work. If 10.5 grams of cyclohexene are actually produced, what is the overall percent yield of our reaction? Show your work.
The following is the dehydration reaction that we would have performed in experiment 19. We would have converted cyclohexanol to cyclohexene.
H3PO4
ILO
Assume that we performed the above reaction starting with 5.7 grams of cyclohexanol.
Calculate the theoretical yield of cyclohexene. Remember that the theoretical yield is the amount that you should get if the reaction goes 100% to completion. Show your work.
If 10.5 grams of cyclohexene are actually produced, what is the overall percent yield of our reaction? Show your work.
Show more…

Added by Emily C.
Instant Answer
Step 1
Step 1: Write the balanced chemical equation for the dehydration reaction of cyclohexanol to cyclohexene: C6H11OH → C6H10 + H2O Show more…
Show all steps