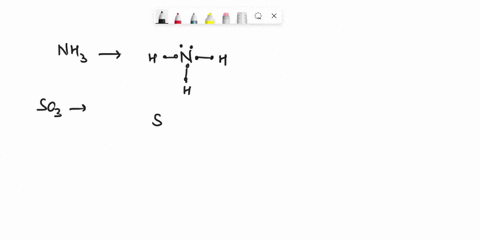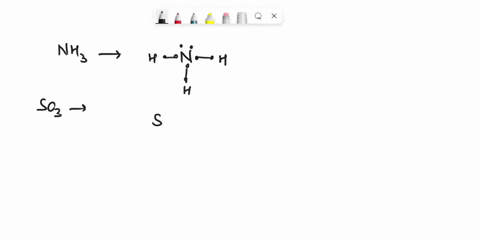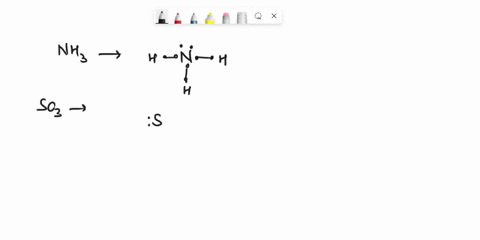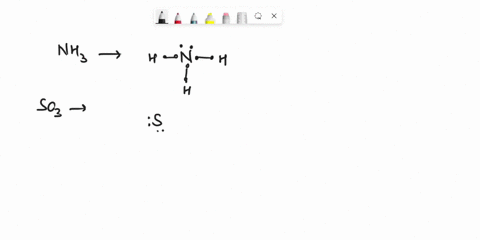
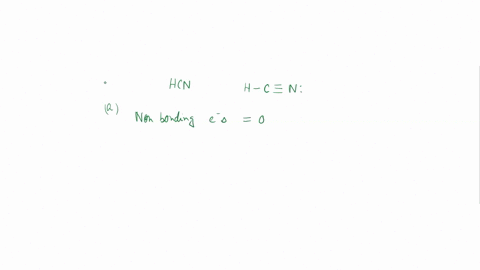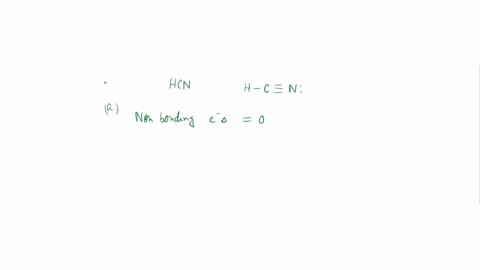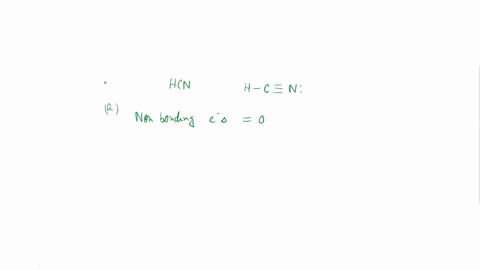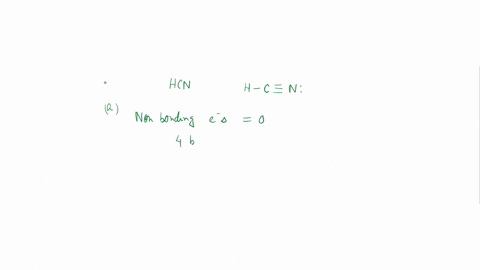Question
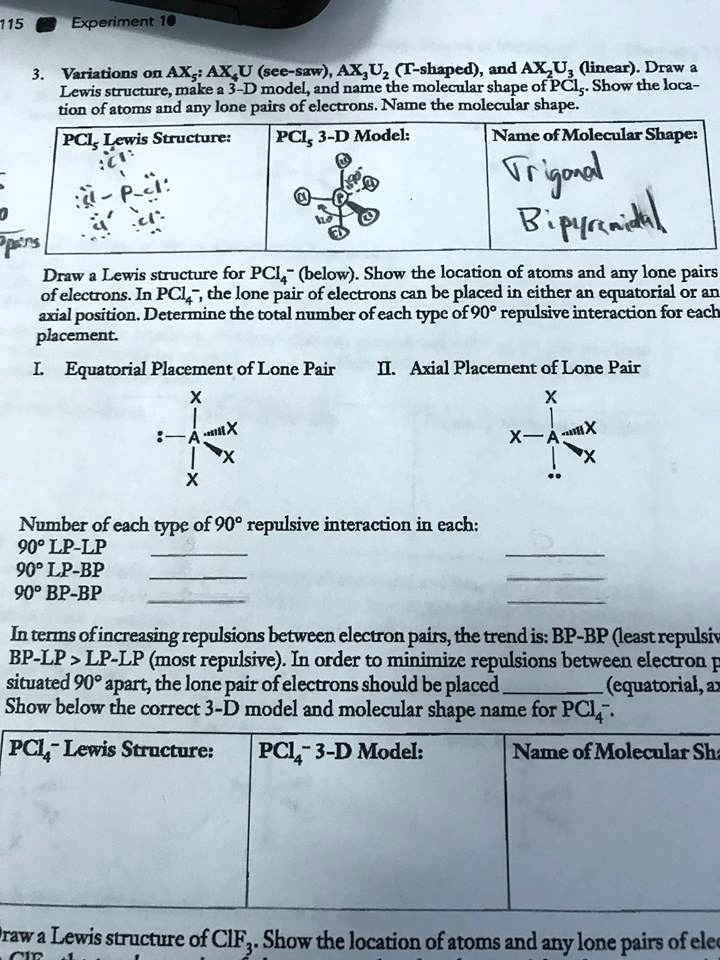
Experiment 10 Variations on AX5; AX4U (see-saw), AX3U2 (T-shaped), and AX2U3 (linear). Draw Lewis structures, make 3-D models, and name the molecular shape of PCl5. Show the location of atoms and any lone pairs of electrons. Name the molecular shape: PCl5 Lewis Structure: PCl5 3-D Model: Name of Molecular Shape: Trigonal Bipyramidal Draw the Lewis structure for PCl3 (below). Show the location of atoms and any lone pairs of electrons. In PCl3, the lone pair of electrons can be placed in either an equatorial or an axial position. Determine the total number of each type of 90° repulsive interaction for each placement: Equatorial Placement of Lone Pair: - LP-LP: 90° - LP-BP: 90° - BP-BP: 90° Axial Placement of Lone Pair: - LP-LP: 90° - LP-BP: 90° - BP-BP: 90° In terms of increasing repulsions between electron pairs, the trend is: BP-BP (least repulsive) < BP-LP < LP-LP (most repulsive). In order to minimize repulsions between electrons situated 90° apart, the lone pair of electrons should be placed in an equatorial position. Show below the correct 3-D model and molecular shape name for PCl3: PCl3 Lewis Structure: PCl3 3-D Model: Name of Molecular Shape: Trigonal Pyramidal Draw a Lewis structure of ClF3. Show the location of atoms and any lone pairs of electrons.
Experiment 10
Variations on AX5; AX4U (see-saw), AX3U2 (T-shaped), and AX2U3 (linear). Draw Lewis structures, make 3-D models, and name the molecular shape of PCl5. Show the location of atoms and any lone pairs of electrons. Name the molecular shape: PCl5
Lewis Structure: PCl5
3-D Model:
Name of Molecular Shape: Trigonal Bipyramidal
Draw the Lewis structure for PCl3 (below). Show the location of atoms and any lone pairs of electrons. In PCl3, the lone pair of electrons can be placed in either an equatorial or an axial position. Determine the total number of each type of 90° repulsive interaction for each placement:
Equatorial Placement of Lone Pair:
- LP-LP: 90°
- LP-BP: 90°
- BP-BP: 90°
Axial Placement of Lone Pair:
- LP-LP: 90°
- LP-BP: 90°
- BP-BP: 90°
In terms of increasing repulsions between electron pairs, the trend is: BP-BP (least repulsive) < BP-LP < LP-LP (most repulsive). In order to minimize repulsions between electrons situated 90° apart, the lone pair of electrons should be placed in an equatorial position.
Show below the correct 3-D model and molecular shape name for PCl3:
PCl3 Lewis Structure: PCl3
3-D Model:
Name of Molecular Shape: Trigonal Pyramidal
Draw a Lewis structure of ClF3. Show the location of atoms and any lone pairs of electrons.
Show more…
Added by Mark S.
Instant Answer
Step 1
Draw the Lewis structure for PCl5: The Lewis structure for PCl5 is: P surrounded by 5 Cl atoms, with single bonds between P and each Cl atom. P has 5 valence electrons and each Cl has 7 valence electrons, making a total of 40 valence electrons. The structure Show more…
Show all steps