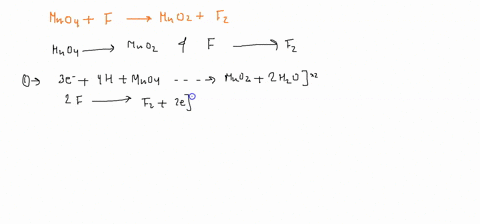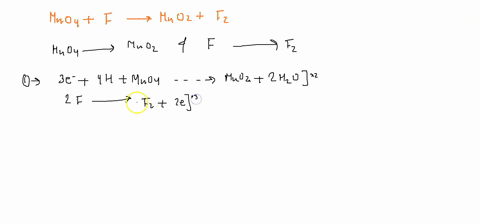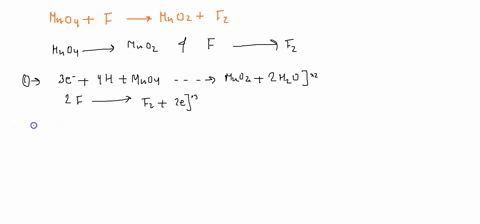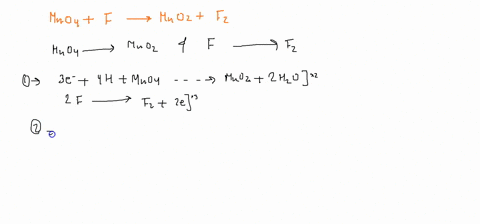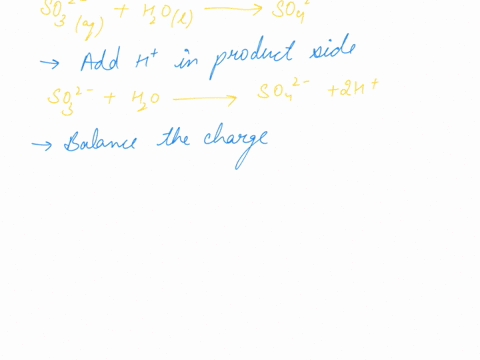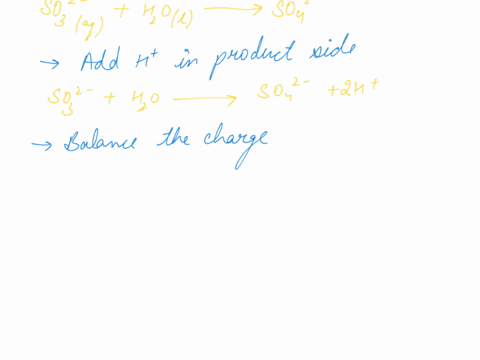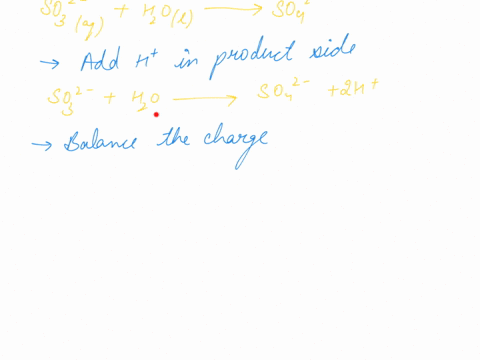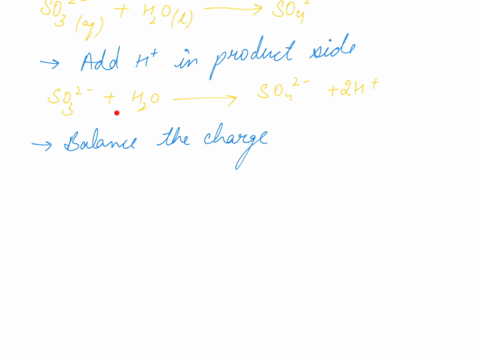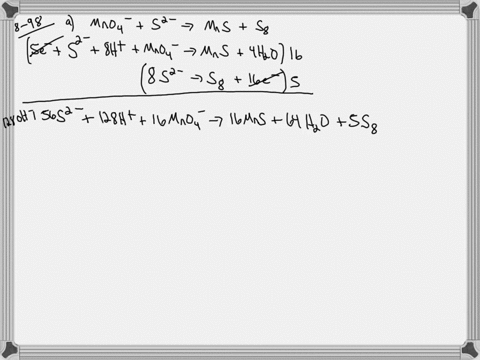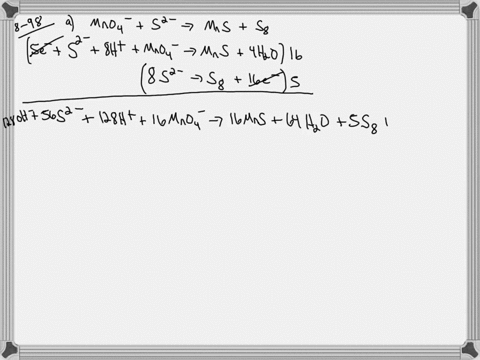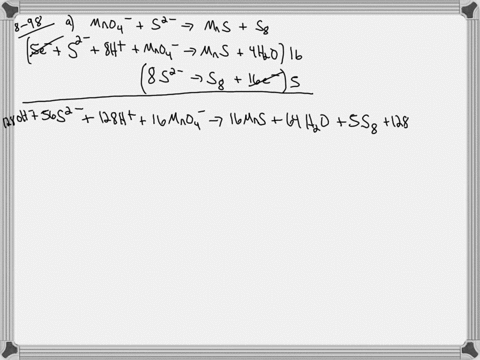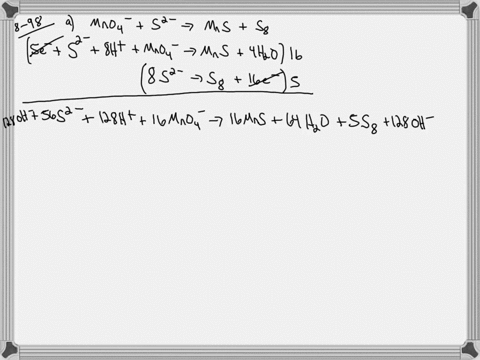Question
When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. The net ionic half-cell reactions are: reduction half-cell reaction: MnO4-(aq) --> Mn2+(aq) Oxidation half-cell reaction: SO32-(aq) --> SO42-(aq) Write and balance the equation for the overall redox reaction (in acidic medium) using the half-reaction method. Show all work.
When sodium sulfite solution is added to acidified potassium
permanganate solution, the purple permanganate solution turns
colourless. The net ionic half-cell reactions are:
reduction half-cell reaction: MnO4-(aq)
--> Mn2+(aq)
Oxidation half-cell reaction: SO32-(aq)
--> SO42-(aq)
Write and balance the equation for the overall redox reaction
(in acidic medium) using the half-reaction method. Show all
work.
Show more…
Added by Sarah R.
Instant Answer
Step 1
- Reduction half-cell reaction: \( \text{MnO}_4^- \rightarrow \text{Mn}^{2+} \) - Oxidation half-cell reaction: \( \text{SO}_3^{2-} \rightarrow \text{SO}_4^{2-} \) Show more…
Show all steps