Abstracts
BACKGROUND: Restless legs syndrome (RLS) is a sensory motor disorder characterized by a distressing urge to move the legs and sometimes also other parts of the body usually accompanied by a marked sense of discomfort or pain in the leg or other affected body part. Many treatments have been used to minimize the discomfort of the disease, among them the anticonvulsant therapy. AIM: This review aims to evaluate the efficacy and safety of anticonvulsant treatment for idiopathic RLS. METHOD: Systematic review of randomized or quasi-randomized, double blind trials on anticonvulsant treatment for RLS. Outcomes: relief of RLS symptoms, subjective and objective sleep quality, quality of life, and adverse events associated with the treatments. RESULTS: A total of 231 patients were randomized in three cross over studies and one parallel study. Three studies with carbamazepine, one with sodium valproate, and one with gabapentin, and they were very heterogeneous so we could not perform a metanalyses. CONCLUSIONS: There is no scientific evidence on RLS treatment with anticonvulsants for clinical practice.
restless legs; anticonvulsant
CONTEXTO: A síndrome das pernas inquietas (SPI ) é uma desordem caracterizada por um impulso de mover as pernas e as vezes outras partes do corpo acompanhado geralmente por desconforto ou da dor nas pernas ou em outra parte afetada. Muitos tratamentos tem sido utilizados para aliviar o desconforto causado pela doença entre eles os anticonvulsivantes. OBJETIVO: Este estudo objetivou avaliar a eficácia e segurança do tratamento da SPI com as drogas anticonvulsivantes. MÉTODO: Revisão sistemática de ensaios clínicos randomizados ou quasi-randomizados, duplo-cegos para o tratamento com anticonvulsivantes para SPI. Desfechos: alívio dos sintomas da SPI, qualidade subjetiva e objetiva do sono, qualidade de vida e efeitos adversos relacionados ao tratamento. RESULTADOS: Um total de 231 pacientes foram randomizados em três estudos cross-over e um estudo paralelo. Três estudos avaliaram a carbamazepina, um estudo avaliou o ácido valpróico e o outro a gabapentina, eles eram muito heterogêneos, o que impossibilitou a metanálise dos resultados. CONCLUSÃO: Não existe evidência científica, que o tratamento da SPI com anticonvulsivantes é eficaz e seguro, para a prática clínica.
pernas inquietas; anticonvulsivantes
VIEWS AND REVIEWS
Anticonvulsants to treat idiopathic restless legs syndrome: systematic review
Anticonvulsivantes para a síndrome das pernas inquietas idiopática: revisão sistemática
Cristiane Fiquene ContiI,II,III; Márcio Moysés de OliveiraI,II,III; Juliana Spelta ValbuzaI,II,III; Lucila Bizari F. PradoI,III; Luciane Bizari Coin de CarvalhoI,III; Gilmar Fernandes do PradoI,III
IDepartment of Emergency Medicine and Evidence Based Medicine (UNIFESP) - Universidade Federal de São Paulo, SP, Brazil (UNIFESP)
IIBrazilian Cochrane Center (UNIFESP) - Universidade Federal de São Paulo, SP, Brazil (UNIFESP)
IIINeuro-Sono, Department of Neurology (UNIFESP) - Universidade Federal de São Paulo, SP, Brazil (UNIFESP)
ABSTRACT
BACKGROUND: Restless legs syndrome (RLS) is a sensory motor disorder characterized by a distressing urge to move the legs and sometimes also other parts of the body usually accompanied by a marked sense of discomfort or pain in the leg or other affected body part. Many treatments have been used to minimize the discomfort of the disease, among them the anticonvulsant therapy.
AIM: This review aims to evaluate the efficacy and safety of anticonvulsant treatment for idiopathic RLS.
METHOD: Systematic review of randomized or quasi-randomized, double blind trials on anticonvulsant treatment for RLS. Outcomes: relief of RLS symptoms, subjective and objective sleep quality, quality of life, and adverse events associated with the treatments.
RESULTS: A total of 231 patients were randomized in three cross over studies and one parallel study. Three studies with carbamazepine, one with sodium valproate, and one with gabapentin, and they were very heterogeneous so we could not perform a metanalyses.
CONCLUSIONS: There is no scientific evidence on RLS treatment with anticonvulsants for clinical practice.
Key words: restless legs, anticonvulsant.
RESUMO
CONTEXTO: A síndrome das pernas inquietas (SPI ) é uma desordem caracterizada por um impulso de mover as pernas e as vezes outras partes do corpo acompanhado geralmente por desconforto ou da dor nas pernas ou em outra parte afetada. Muitos tratamentos tem sido utilizados para aliviar o desconforto causado pela doença entre eles os anticonvulsivantes.
OBJETIVO: Este estudo objetivou avaliar a eficácia e segurança do tratamento da SPI com as drogas anticonvulsivantes.
MÉTODO: Revisão sistemática de ensaios clínicos randomizados ou quasi-randomizados, duplo-cegos para o tratamento com anticonvulsivantes para SPI. Desfechos: alívio dos sintomas da SPI, qualidade subjetiva e objetiva do sono, qualidade de vida e efeitos adversos relacionados ao tratamento.
RESULTADOS: Um total de 231 pacientes foram randomizados em três estudos cross-over e um estudo paralelo. Três estudos avaliaram a carbamazepina, um estudo avaliou o ácido valpróico e o outro a gabapentina, eles eram muito heterogêneos, o que impossibilitou a metanálise dos resultados.
CONCLUSÃO: Não existe evidência científica, que o tratamento da SPI com anticonvulsivantes é eficaz e seguro, para a prática clínica.
Palavras-chave: pernas inquietas, anticonvulsivantes.
Restless legs syndrome (RLS) is a sensory motor disorder characterized by a distressing urge to move the legs and sometimes also other part of the body usually accompanied by a marked sense of discomfort or pain in the leg or other affected body part1. The prevalence of RLS is estimated to be 5% to 15% in adults and it is more common in women2,3, and was firstly reported in Brazil by Mattos Pimenta4. RLS may be either idiopathic (primary RLS, which often has a familial component) or secondary, occurring with other medical conditions, particularly iron deficiency anaemia, pregnancy, or end-stage renal disease5.
The most common pharmacological agents used in clinical practice for the treatment of RLS are levodopa, dopamine agonists, opioids, benzodiazepines and anticonvulsants1,6. Anticonvulsants are generally structural analogues of g-aminobutiric acid (GABA) but in many cases the precise receptor(s) and biochemical function still remain unknown. It is suggested that novel receptor binding sites and an accumulation of GABA in different regions of the brain might be responsible for its effects in RLS7. Its psychotropic and analgesics effects may be exerted, at least indirectly, through other neurotransmitters. In vivo the gabapentin antagonizes 3,4-diamopyridine-induced release of dopamine, nor-epinefrine and serotonin in the rat hippocampus, mesolimbic and striatal regions. These neurotransmitters, and in particular dopamine, are probably involved in the pathophysiology of RLS7-9.
Although anticonvulsants seems especially useful for treating mild to moderate RLS, particularly in patients reporting pain, the effectiveness of this class of drugs in the treatment of RLS is still controversial. In this clinical setting was relevant to conduct a systematic review to evaluate the clinical benefit of anticonvulsants for RLS.
METHOD
Inclusion criteria
Study design: Parallel and cross-over randomized and quasi-randomized controlled trials; Participants: Patients who meet any clinical criteria for idiopathic RLS10,11; Exclusion criteria: Studies with secondary form of RLS such as metabolic, neuropathic or renal disease; Types of interventions: All anticonvulsants drugs used for treatment of RLS; Comparison groups include: Placebo; no intervention and other category of drugs; Outcomes measures: Relief of RLS symptoms as measured by the International RLS Study Group Rating Scale for RLS (IRLSSG Rating Scale) was the primary outcome12. Secondary outcomes were adverse events associated with the treatments (including augmentation); subjective sleep quality (any description about sleep quality, i.e., feeling like wellbeing, tiredness reduction); objective sleep quality measured by night polissomnografy (sleep efficiency, total sleep time, arousal index, PLMS index). The ethics committee of Universidade Federal de São Paulo/hospital São Paulo approved the study.
The electronic search
The search strategies were run on December 2007 using the following terms and their synonymous: restless legs syndrome, Ekbom syndrome, periodic leg movement, periodic limb movement, PLM, PLMS, PLMD, sindrome de las piernas inquietas, síndrome das pernas inquietas, sindrome delle gambe senza riposo, syndrome des jambs sans repos, idiopathischen restless-legs-syndrom. The search for trials was carried out through The Cochrane Library, Medline, Pubmed, Lilacs, Embase and Scielo. Besides the most traditional electronic databases, other sources were also considered: thesis indexed at BIREME/PAHO-WHO (Biblioteca Regional Medicina/Panamerican Health Organization of the World Health Organization); reference list of all recovered trials; additional information asked for the authors of primary studies by electronic mail.
Methodological quality of included studies
The studies were judged according to Cochrane handbook based on allocation concealment (randomization), blinding (performance and detection bias), and attrition bias.
Data analysis
Data synthesis and analysis was performed using the Cochrane Review Manager software, RevMan version 4.2.8. Dichotomous data(adverse events) were expressed as relative risk (RR). Weighted mean difference (WMD) was used when continuous data were available from included studies. We included crossover study designs if the reported results had their within-patients variation, as given by paired tests13. For both dichotomous and continuous data, we used 95% confidence intervals (CI). The statistical analysis using random effect model14 was used to estimate the treatment effects as compared to placebo. Statistical significance was considered when p<0.05. heterogeneity between estimated effects across studies was analyzed using the inconsistency test (I2), which describes the percentage of the variability in effect estimates due to heterogeneity rather than sampling error15. Statistical heterogeneity was assumed in case I2>50%. According to the type of information available in the articles or by contacting the authors of primary studies, the results were pooled based on either endpoint or change from baseline as well as on subcategories of medication and time length of treatment.
RESULTS
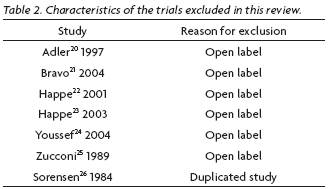
We identified 1028 studies related to pharmacological treatment for RLS, among them eleven trials published related to anticonvulsant therapy. Four randomized clinical trials in English were included in this review for they fulfilled the inclusion criteria (Table 1). Seven trials were excluded (Table 2), six trials were not randomized ones (open-label), and one trial was excluded because it was a duplicated published.
A total of 231 patients were randomized in crossover and parallel design studies. Fifty patients were randomized in crossover studies (6 patients received carbamazepine, 20 received valproic acid, and 24 received gabapentin), and out of 181 patients were randomized in the parallel-arm trial (84 received carbamazepine, and 90 received placebo).
We found several subjective measures (visual analogue scales, patients global impression, clinical global impression, Pittsburg sleep quality index, IRLSSG rating scale) and objective measures (polysomnographic measures) used in these studies such as describe below.
Borregueiro16
IRLSSG rating scale - The patients with gabapentin compared to placebo reported that had a greater decrease in IRLSSG rating scale (WMD: -8.40; 95% cI -12.00 to -4.80; p<0.001);
Patients global impression - There was no improvement in the scores of patients global impression (WMD: 20.5; 95% CI 13.30 to 27.70; p<0.0001);
Clinical global impression - There was a lower and not statistically significant improvement in the scores of clinical global impression (WMD: -1.10; 95% cI -1.93 to -0.27; p<0.01);
Pittsburg sleep quality index - There was a lower improvement in sleep quality (WMD: -2.90; 95% IC -4.01 to -1.79; p<0.001);
Visual analogue scale - The results were not statistically significant (p=NS);
Polissomnographic measures - The patients with gabapentin compared to placebo reported a reduced PLMI (WMD: -9.70; 95% cI -18.84 to -0.56; p<0.05), showed a not statistical significant improvement of PLMAI (WMD: -1.10; 95%cI -4.70 to 2.50; p=NS), increased the total sleep time (WMD: -0.50; 95% cI -0.77 to -0.23; p=0.01), sleep efficiency (WMD: -9.80; 95% cI -13.95 to -5.65; p<0.0001), stage 1 (WMD: -22.80; 95% cI -41.92 to -3.68; p<0.05), and slow wave sleep (WMD: -22.30; 95% cI -42.25 to -2.35; p<0.05). The others measures, sleep latency (p=NS), stage 2 (p=NS), REM stage (p=NS), REM sleep latency (p=NS), and arousal index (p=NS) were not statistically significant (*NS= no significant).
Adverse events - The side effects occur in 48% of patients taking gabapentin, malaise and abdominal pain were the most common. No cases of augmentation phenomenon were described.
Telstald17
Subjective measures - In subjective analyses reported by patients both placebo and carbamazepine decreased the number of attacks, but carbamazepine was more effective than placebo (p=0.02).
Adverse events - The side effects were not described in these study and seven drop outs were described (reason not related to the treatment).
Lundval18
Subjective measures - The study reported in subjective measures improvement in 50% of patients, and other 50% reported insufficient improvement. No statistical analyses were performed in this study.
Adverse events - The side effects reported were mild gastritis, sweating, dizziness and vomiting and two drop outs were described (reason by the lack of the effect of carbamazepine).
Eisensehr19
Subjective measures - Slow-release valproic acid significantly decreased the subjectively rated intensity of RLS complaints (WMD: -1.70; 95% cI -3.02 to -0.38, p=0.022) and duration of symptoms (WMD: -92.30; 95% cI -231.76 to 47.06; p=0.007) compared to placebo.
Polissomnographic measures - Total sleep time (p=NS), wake after sleep onset (p=NS), PLMAI (p=NS), stage 1 (p=NS), stage 2 (p=NS), stage3/4 (p=NS), stage REM (p=NS), PLMI (p=NS), and arousal index (p=NS) were not statistically significant.
Adverse events - The side effects headache, drowsiness, and difficulties falling asleep were the most common adverse events and there were no drop outs.
DISCUSSION
Overall the methodological quality of the studies was considered as adequate (A) in two studies (Borregueiro16; Eisensehr19), and unclear (B) in the others two studies (Telstald17; Lundval18). On the other hand, almost all included studies were classified as "low risk" of systematic error, according to methodological quality of included studies.
The methodological quality of the carbamazepine included studies (Telstald17; Lundval18) was considered poor, where data and methods reported in these articles were not described by the authors with sufficient details, even after contact by e-mail we also could not retrieve further information from the authors. however the studies about slow-release valproic acid (Eisensehr19) and gabapentin (Borregueiro16) showed good methodological quality and the results for continuous outcome measures were satisfactory. But their short-term follow up, which ranged from three to twelve weeks, was not possible to estimate the efficacy of treatment before doing long-term evaluations.
It may be a source of bias that different treatment sequences were not separated by washout periods. however, in Eisensehr19 study outcome variables were measured at the end of each treatment sequence, when (based on the half lives of the drugs) there was probably no effect of the drug taken before. A washout period was present in Borregueiro16 study, the one week washout period was probably based on the half life of the drug either, but there is no evidence that its clinical action is short-lasting, and its duration seems not sufficient to avoid a carry over effect of gabapentin.
Overall, the drugs were well-tolerated, except in Borregueiro16 study, when 48% of patients had side effects taking gabapentin but no patients dropped out because of them.
Augmentation syndrome, which consists of an abnormally severe pattern of RLS, is a common reason for changing medications27 and an important clinical problem noted on other therapies, such as levodopa and dopaminergic agonists. It is important to note that no cases of augmentation were reported with anticonvulsant therapy in a short-term follow up. The present reviewers advise physicians who are in charge of treatment for RLS patients to be aware of the augmentation syndrome in a long-term follow up.
There is no evidence that pharmacological treatment for RLS with anticonvulsant is effective and safe. Future trials should follow internationally published guidelines for reporting trials. Well designed randomized clinical trials are needed to assess the efficacy and safety of anticovulsant therapy for Restless legs syndrome.
Received 27 July 2007, received in final form 17 March 2008. Accepted 11 April 2008.
Dr. Gilmar Fernandes do Prado - Rua Claudio Rossi 404 - 01547-050 São Paulo SP - Brasil. E-mail: [email protected]
References
- 1. Trenkwalder C, Paulus W, Walters AS. The restless legs syndrome review. Lancet Neurol 2005;4:465-475.
- 2. Picchietti D, Winkelman JW. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep 2005;28:891-898.
- 3. Teive HA, Quadros A, Barros FC, Werneck LC. Worsening of autosomal dominant restless legs syndrome after use of mirtazapine: case report. Arq Neuropsiquiatr 2002;60:1025-1029.
- 4. Pimenta AM. Pernas inquietas: estudo de uma família. Comunicações correlatas e livres do V Congresso Brasileiro de Neurologia. Arq Neuropsiquiatr 1972;resumos:100.
- 5. Goffredo GS Filho, Gorini CC, Purysko AS, Silva HC, Elias IE. Restless legs syndrome in patients on chronic hemodialysis in a Brazilian city: frequency, biochemical findings and comorbidities. Arq Neuropsiquiatr 2003;61:723-727.
- 6. Grupo Brasileiro de Estudos em Síndrome das Pernas Inquietas. Restless legs syndrome: diagnosis and treatment. Opinion of Brazilian experts. Arq Neuropsiquiatr 2007;65:721-727.
- 7. Ondo W,Jankovic J. Restless legs syndrome: clinicoetiologic correlates. Neurology 1996;47:1435-1441.
- 8. Allen RP. Dopamine and iron in the pathophysiology of restless legs syndrome. Sleep Med 2004;5:385-391
- 9. Pugsley TA, Whetzel SZ, Dooley DJ. Reduction of 3,4-diaminopyridine-induced biogenic amine synthesis and release in rat brain by gabapentin. Psychopharmacology 1998;137:74-80.
- 10. American Sleep Disorders Association. The international classification of sleep disorders: diagnostic and coding manual. Revised Edition. Rochester, MN: American Sleep Disorders Association 1997
- 11. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003;4:101-109.
- 12. The International Restless Legs Syndrome Study Group. Validation of The International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 2003;4:121-132.
- 13. Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140-149.
- 14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7:177-188.
- 15. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.
- 16. Borreguero GD, Larrosa O, LlaveY, VergerK, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin. Neurology 2002;59:1573-1579.
- 17. Testald W, Sorensen O, Larsen S, Lillevold PE, Stensrud P, Hanssen RN. Treatment of restless legs syndrome with carbamazepine: a double blind study. BMJ 1984;288:444-446.
- 18. Lundvall O, Abom PE, Holm R. Carbmazepine in Restless Legs Syndrome. Eur J Clin Pharmacol 1983;25:323-324.
- 19. Eisensehr I, Ehrenberg BL, Rogge Solti S, Noachtar S. Treatment of idiopathic restless legs syndrome (RLS) with slow-release valproic acid compared with slow-release levodopa/benserazid. J Neurol 2004;5:579-583.
- 20. Adler CH. Clin Neuropharmacol 1997;20:148-151.
- 21. Bravo AP. Utilidad del topiramato en el tratamiento del síndrome de piernas inquietas. Actas Esp Psiquiatr 2004;32:132-137.
- 22. Happe S, Klösch G, Saletu B, Zeitlhofer J. Treatment of idiopathic restless legs syndrome (RLS) with gabapentin. Neurology 2001;57:1717-1719.
- 23. Happe S, Saulter C, Klosh G, Saletu B. Gabapentin versus ropinirole in the treatment of idiophatic restless legs syndrome. Neuropsychobiology 2003;48:82-86.
- 24. Youssef EA, Wagner ML, Martinez JO, Hening W. Pilot trial of lamotrigine in the restless legs syndrome. Sleep Med 2005;6:89.
- 25. Zuconni M, Coccagna G, Petronelli R, Gerardi R, Mondini S, Cirignotta F. Nocturnal myoclonus in restless legs syndrome effect of carbamazepine treatment. Functional Neurol 1989;4:263-271.
- 26. Sorensen O, Testald W. Carbamazepin (Tegretol) ved restless legs syndrome. Tidsskr Nor Laegeforen 1984;104:2093-2095.
- 27. Allen RP,Earley CJ. Augmentation of the restless legs syndrome with carbidopa levodopa. Sleep 1996;14:629-650.
Publication Dates
-
Publication in this collection
29 Sept 2008 -
Date of issue
June 2008
History
-
Accepted
11 Apr 2008 -
Received
27 July 2007

 Anticonvulsants to treat idiopathic restless legs syndrome: systematic review
Anticonvulsants to treat idiopathic restless legs syndrome: systematic review