Quinolones
- 2. The Quinolones The Quinolones The quinolones are really not antibiotics. By definition, an antibiotic means something that's produced by another living substance. That's the real definition. We have a lot of chemotherapeutic antimicrobials that have activity against organisms for which we loosely use the term antibiotic, but they're really chemicals.
- 3. History A group of synthetic antibacterial agents mainly effective against G-ve Nalidixic acid first member introduced in 1964 for urinary and GIT infections Its congeners Oxolinic acid and rosoxacin with more potency in 1970s Second generation called fluoroquinolones with extended spectrum and systemic effects in 1980s Since then many synthesized with useful spectrum.
- 4. Chemistry of Quinolones They are synthetic fluorinated analogs of nalidixic acid.
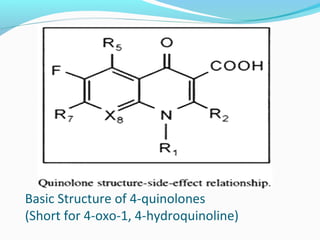
- 5. Basic Structure of 4-quinolones (Short for 4-oxo-1, 4-hydroquinoline)
- 6. The carboxyl group at position 3 and ketone group at position 4 are essential for antimicrobial activity. Substitution at position 6 with a fluorine moiety markedly increase antibacterial activity against G+ve, G-ve , Mycoplasma and chlamydia.. Addition of a piperazine ring at position 7 on fluoroquinolones increases tissue and bacterial penetration and improves spectrum of activity to include Pseudomonas ( e.g. Ciprofloxacin, Enrofloxacin
- 8. Generations Researchers divide the quinolones into generations based on their antibacterial spectrum. The earlier-generation agents are, in general, more narrow-spectrum than the later ones, but no standard is employed to determine which drug belongs to which generation. The only universal standard applied is the grouping of the non fluorinated drugs found within this class (quinolones) within the 'first-generation' heading.
- 9. Classification of Quinolones First-generation cinoxacin flumequine nalidixic acid oxolinic acid piromidic acid pipemidic acid rosoxacin
- 10. Second-generation The second-generation class is sometimes subdivided into "Class 1" and "Class 2". Ciprofloxacin enoxacin fleroxacin lomefloxacin nadifloxacin norfloxacin ofloxacin (only as ophthalmic in the United States) pefloxacin
- 11. Third-generation Unlike the first- and second-generations, the third-generation is active against streptococci. balofloxacin grepafloxacin levofloxacin pazufloxacin sparfloxacin temafloxacin tosufloxacin
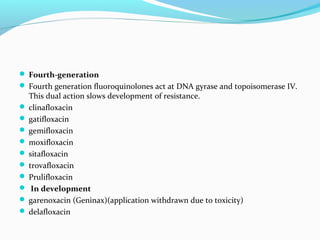
- 12. Fourth-generation Fourth generation fluoroquinolones act at DNA gyrase and topoisomerase IV. This dual action slows development of resistance. clinafloxacin gatifloxacin gemifloxacin moxifloxacin sitafloxacin trovafloxacin Prulifloxacin In development garenoxacin (Geninax)(application withdrawn due to toxicity) delafloxacin
- 13. Antimicrobial effects of Quinolones a. Broad spectrum a. First-generation, G- aerobic bacteria; b. Second-generation, more activity against G- aerobic bacteria; c. Third-generation, fluoroquinolones, possess excellent G- activity & moderate to good activity against G+ bacteria, anaerobes,
- 14. Summary of antimicrobial spectrum of quinolones
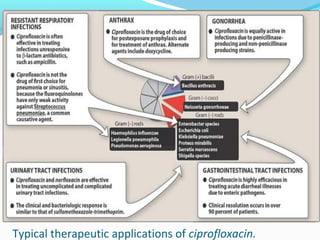
- 15. Typical therapeutic applications of ciprofloxacin.
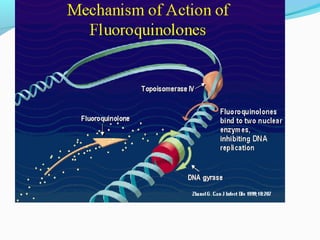
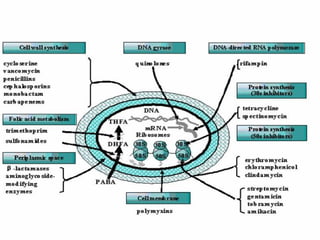
- 16. Mechanism of action 1.They block bacterial DNA synthesis by inhibiting bacterial topoisomerase (Ⅱ DNA gyrase) and topoisomerase .Ⅳ 2. Inhibition of DNA gyrase prevents the relaxation of positively supercoiled DNA that is required for normal transcription and replication. 3.Inhibiton of topoisomerase probably interferes withⅣ separation of replicated chromosomal DNA into the respective daughter cells during cell division.
- 18. Mechanism of action 4.The gyrase is composed of two A subunits and two B subunits. The A subunits can cut one of double strands of the DNA .This is an ATP- dependent reaction. The energy is provided by B units.
- 19. Mechanism of action 5.Quinolones is an inhibitor of A subunits. Therefore, the action of gyrase is inhibited and DNA replication or transcription is blocked as result of the death of bacteria. 6. Novobiocin is an inhibitor of the B subunit of DNA gyrase and is active mainly against G+ bacteria.
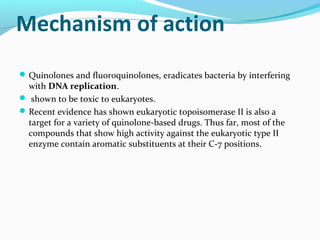
- 20. Mechanism of action Quinolones and fluoroquinolones, eradicates bacteria by interfering with DNA replication. shown to be toxic to eukaryotes. Recent evidence has shown eukaryotic topoisomerase II is also a target for a variety of quinolone-based drugs. Thus far, most of the compounds that show high activity against the eukaryotic type II enzyme contain aromatic substituents at their C-7 positions.
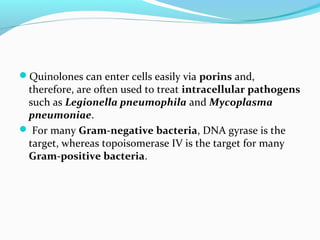
- 21. Quinolones can enter cells easily via porins and, therefore, are often used to treat intracellular pathogens such as Legionella pneumophila and Mycoplasma pneumoniae. For many Gram-negative bacteria, DNA gyrase is the target, whereas topoisomerase IV is the target for many Gram-positive bacteria.
- 23. Resistance to Quinolones 1. Due to a. One or more point mutations in the quinolone binding region of the target enzyme b. A change in the permeability of the organism 2. DNA gyrase is the primary target in E coli, with single- step mutants exhibiting amino acid substitution in the A subunit of gyrase. 3. Topoisomerase is a secondary target in E coli that isⅣ altered in mutants expressing higher levels of resistance.
- 24. Resistance to Quinolones 4. In staphylococci and streptococci, the situation is reversed, topoisomerase is the primary target,Ⅳ and gyrase is the secondary target. 5. Resistance to one fluoroquinolone, particularly if of high level, generally confers cross-resistance to all other members of this class.
- 25. Pharmacokinetics of Quinolones a. Oral given, well absorbed, be impaired by divalent cations, including those in antacids, b. Distributed widely in body fluids and tissues, pass placenta reach to the fetus, c. Biotransformation of the drugs in the liver d. Most eliminated by renal, either tubular secretion or glomerular filtration.
- 26. Effect of dietary calcium on the absorption of ciprofloxaxin
- 27. Clinical uses of Quinolones 1. Nalidixic acid is only second-line drug treating urinary infection with gram- negative bacilli (Bacillus coli, Bacillus proteus , etc). 2. Pipemidic acid not only is used treating infection of urinary tract but also treating intestinal and biliary tract infection with sensitive bacteria.
- 28. Clinical uses of Quinolones 3. Fluoroquinolones are extensively used treating general infection. a. urinary tract infections, even when caused by multifrug-resistant bacteria, b. Intestinal and biliary tract infections c. Soft tissue infections d. Bone, joint and in intra-abdominal e. Respiratory tract infections
- 29. Clinical uses of Quinolones Ciprofloxacin and ofloxacin are effective for gonococcal infection, including disseminated disease. They are occasionally used for treatment of tuberculosis and atypical mycobacterial infections. They are suitable for eradication of meningococci from carriers.
- 30. Clinical uses of Quinolones Ofloxacin is effective for chlamydial urethritis or cervicitis. Ciprofloxacin is a second-line agent for legionellosis. Levofloxacin, sparfloxacin are used for treatment of upper and lower respiratory tract infections.
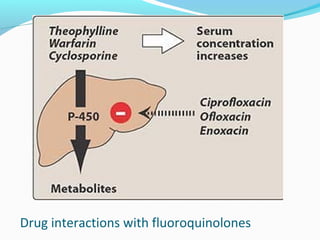
- 31. Drug interactions with fluoroquinolones
- 32. Adverse effects of Quinolones a. The most common effects are nausea, vomiting, and diarrhea. b. Headache, dizziness, insomnia, skin rash, occasionally. c. Liver toxicity is rarely for trovafloxacin. d. Photosensitivity occurs with lomefloxacin and pefloxacin.
- 33. Adverse effects of Quinolones e. Fluoroquinolones may damage growing cartilage and cause an arthropathy. They are not used in patients under 18 years of age. The arthropathy is reversible. Since fluoroquinolones are excreted in breast milk, they are contraindicated for nursing mothers.