Coagulation and flocculation
- 1. Presentation on Coagulation and Flocculation Presented by: Mr. Muhammad Azeem Presented to: Engr. Muhammad Sulaiman 1
- 2. Outlines Purpose of Techniques Coagulation Coagulants Selection of Coagulant Amount of Coagulant Flocculation Types of Flocculation Flocculation Mixers Conclusion 2
- 3. Purpose Settling of stabilized particles Destabilization of colloidal particles Increase the density of colloidal particles Reduction in repulsive forces between particles Diminish the turbidity of water 3
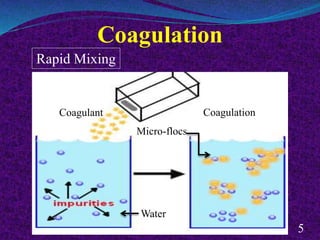
- 4. Coagulation Coagulation is the destabilization of colloidal and fine suspended solids by adding a chemical coagulant such as alum in the water. Neutralization of negatively charged particles Micro-flocs formation 4 + Coagulant Coagulation Tank Destabilization of colloids
- 6. Commonly Used Coagulants Coagulant Formula Aluminum Sulfate Al2(SO4)3 · 14H2O Sodium Aluminate Na2Al2O4 Aluminum Chloride AlCl3 Ferric Chloride FeCl3 Ferric Sulfate Fe2(SO4)3 6
- 7. Selection of Coagulant A coagulant is selected on the basis of: Type of Coagulant (anionic, cationic) Molecular Weight (high) Charge Density (high) Toxicity (non toxic) Solubility (insoluble in neutral pH range) 7
- 8. Type of Coagulant Cationic: In the process of coagulation Positively charged coagulant Anionic: In the process of flocculation Negatively charged coagulant used as flocculent 8
- 10. Jar Test Jar test is a laboratory procedure to determine the optimum pH and the optimum coagulant dose A jar test simulates the coagulation and flocculation processes Fill the jars with raw water sample (500 or 1000 mL), usually 6 jars 10 Determination of optimum pH:
- 11. Jar Test - Optimum pH Adjust pH of the jars while mixing using H2SO4 or NaOH/lime (pH: 5.0; 5.5; 6.0; 6.5; 7.0; 7.5) Add same dose of the selected coagulant (alum or iron) to each jar (coagulant dose: 5 or 10 mg/L) Rapid mix each jar at 100 to 150 rpm for 1 minute. The rapid mix helps to disperse the coagulant throughout each container 11
- 12. Jar Test - Optimum pH Reduce the stirring speed to 25 to 30 rpm and continue mixing for 15 to 20 mins. This slower mixing speed helps to promote flocs formation by enhancing particle collisions, which lead to larger flocs Turn off the mixers and allow flocs to settle for 30 to 45 mins Measure the final residual turbidity in each jar Plot residual turbidity against pH 12
- 13. Jar Test - Optimum pH 13Optimum pH: 6.3 0 5 10 15 20 4.5 5 5.5 6 6.5 7 7.5 8 TurbidityRemaining pH Graph b/w Turbidity Remaining and pH
- 14. Jar Test - Optimum Dose Optimum coagulant dose: Repeat all the previous steps This time adjust pH of all jars at optimum (6.3 found from first test) while mixing using H2SO4 or NaOH/lime Add different doses of the selected coagulant to each jar (Coagulant dose: 5; 7; 10; 12; 15; 20 mg/L) 14
- 15. Jar Test - Optimum Dose Rapid mix each jar at 100 to 150 rpm for 1 minute. The rapid mix helps to disperse the coagulant throughout each container. Reduce the stirring speed to 25 to 30 rpm for 15 to 20 mins Turn off the mixers and allow flocs to settle for 30 to 45 mins Then measure the final residual turbidity in each jar Plot residual turbidity against coagulant dose 15
- 16. Jar Test - Optimum Dose 16Optimum Dose: 11.2 mg/L 0 5 10 15 20 0 2 4 6 8 10 12 14 16 18 20 22 24 TurbidityRemaining Coagulant Dose (mg/L) Graph b/w Turbidity Remaining and Coagulant Dose
- 17. Flocculation Flocculation is the aggregation of micro-flocs into macro-flocs to enhance their settling by gravity sedimentation. Anions are used as bridging media between micro-flocs. + Flocculent 17 Micro-flocs Macro-flocs ….
- 20. Types of Flocculation Micro Flocculation Macro Flocculation Micro Flocculation: Due to random motion of fluid molecules Size range from 0.001 to about 1 µm 20 Randomly Moving Molecules Flocs Formation
- 21. Types of Flocculation Macro Flocculation: Size greater than 1 µm 21 Macro Flocculation Induced Velocity Gradients Differential Settling
- 22. Induced Velocity Gradients Due to change in velocity of particles Fast moving particles overtake slow moving particles Large particle formation due to stickiness of particles 22 L.P S.P Flocs Formation
- 23. Differential Settling Large particles overtake small particles Settling through gravity 23 L.P S.P Flocs Formation
- 24. Polymeric Flocculation 24 + Polymer Micro-flocs Macro-flocs Polymeric Flocculation
- 25. Hydraulic Flocculation 25 Due to the flow of water Horizontally Baffled Tank Vertically Baffled Tank Horizontally Baffled Tank: H2O H2O Horizontally Baffled Tank Baffle
- 26. Hydraulic Flocculation Vertically Baffled Tank: Vertically Baffled Tank 26 H2O H2O Baffle
- 27. Types of Flocculation Mixers Following are the types of flocculation mixers: Static Mixer Paddle Mixer Turbine Mixer Static Mixer: Static vanes or baffles for mixing Water passes through vanes or baffles Horizontally baffled tank (slide no. 25) 27
- 28. Paddle Mixer 28 Slow movement of paddles cause flocs formation PaddlesMoving Shaft
- 29. Turbine Mixer Mixing due to the movement of blades 29
- 30. Conclusion A coagulant is necessary to destabilize the stable impurities of water Flocculation is necessary to develop the macro- flocs of impurities that are settled down easily 30
- 31. References Wastewater Engineering Treatment by Metcalf Eddy Water and Waste Water Engineering by Mackenzie L. Davis Unit Operations and Processes in Environmental Engineering 31
- 32. Thank You 32