Blood/White Blood Cells/Fluid Connective tissue
- 1. BLOOD White Blood cells, Leukemia
- 2. Human Physiology-I Md. Saiful Islam Dept. of Pharmaceutical Sciences North South University Facebook Group: Pharmacy Universe
- 3. Blood is a specialized fluid connective tissue consisting of some cells suspended in a liquid intercellular substance known as plasma.
- 4. Composition of blood Blood cells: 1. Erythrocyte/RBC 2. Leukocyte/WBC 3. Thrombocyte/Platelet Blood is composed of mainly two parts: • Plasma • Blood cells
- 6. Genesis of Blood Cells
- 7. Leukocytes (Greek: “White Hollows”) • All WBCs (leukocytes) have a nucleus and no hemoglobin. • Protect body against microorganisms and remove dead cells and debris. • Their average total number is 6000 to 8000 per mm3. • Granular or agranular classification based on presence of cytoplasmic granules made visible by staining. • Types & Functions • Granulocytes – Neutrophils: Small phagocytic cells – Eosinophils: Reduce inflammation – Basophils: Release histamine and increase inflammatory response • Agranulocytes – Lymphocytes: Immunity – Monocytes: Become macrophages
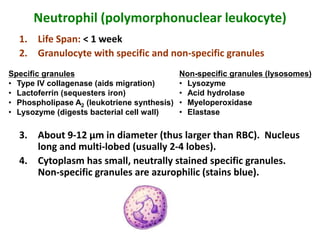
- 8. Neutrophil (polymorphonuclear leukocyte) 1. Life Span: < 1 week 2. Granulocyte with specific and non-specific granules 3. About 9-12 µm in diameter (thus larger than RBC). Nucleus long and multi-lobed (usually 2-4 lobes). 4. Cytoplasm has small, neutrally stained specific granules. Non-specific granules are azurophilic (stains blue). Specific granules • Type IV collagenase (aids migration) • Lactoferrin (sequesters iron) • Phospholipase A2 (leukotriene synthesis) • Lysozyme (digests bacterial cell wall) Non-specific granules (lysosomes) • Lysozyme • Acid hydrolase • Myeloperoxidase • Elastase
- 9. Chemotaxis (from chemo- + taxis) is the movement in response to a chemical stimulus. 5. Function: Primarily antibacterial • Give fastest response of all WBC to bacteria and parasites. • Neutrophils leave the blood and follow chemotaxic signals to sites of wounding or other inflammation, and phagocytose foreign agents such as bacteria. Pus is composed largely of dead neutrophils. • Direct actions against bacteria - release lysozymes which destroy/digest bacteria - release defensin proteins that act like antibiotics - release strong oxidants (bleach-like, strong chemicals ) that destroy bacteria
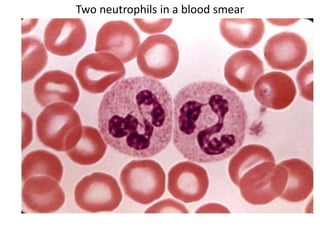
- 10. Two neutrophils in a blood smear
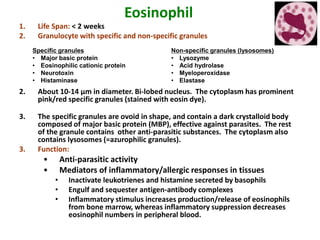
- 11. Eosinophil 1. Life Span: < 2 weeks 2. Granulocyte with specific and non-specific granules 2. About 10-14 µm in diameter. Bi-lobed nucleus. The cytoplasm has prominent pink/red specific granules (stained with eosin dye). 3. The specific granules are ovoid in shape, and contain a dark crystalloid body composed of major basic protein (MBP), effective against parasites. The rest of the granule contains other anti-parasitic substances. The cytoplasm also contains lysosomes (=azurophilic granules). 3. Function: • Anti-parasitic activity • Mediators of inflammatory/allergic responses in tissues • Inactivate leukotrienes and histamine secreted by basophils • Engulf and sequester antigen-antibody complexes • Inflammatory stimulus increases production/release of eosinophils from bone marrow, whereas inflammatory suppression decreases eosinophil numbers in peripheral blood. Specific granules • Major basic protein • Eosinophilic cationic protein • Neurotoxin • Histaminase Non-specific granules (lysosomes) • Lysozyme • Acid hydrolase • Myeloperoxidase • Elastase
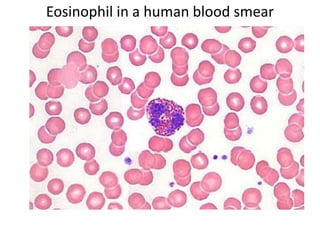
- 12. Eosinophil in a human blood smear
- 13. Basophil 1. Life Span: 1-2 years (?) 2. Granulocyte with specific and non-specific granules 2. About 8-10 µm in diameter. The cytoplasm contains large, purple/black specific granules (stained with the basic dye) that are larger but not as numerous as those of eosinophils. The nucleus is usually bi-lobed, but usually is partially obscured by granules, which can lie over it. 3. The specific granules vary in size and shape, and have occasional myelin figures (usually formed from phospholipids). The cytoplasm also has some lysosomes (=azurophilic granules). 4. Function: Allergies and anaphylaxis (hypersensitivity reaction) • Binding of antigens to membrane-bound IgE antibodies induces degranulation of specific granules, which leads to allergic reaction. • In hypersensitivity reaction, widespread vasodilation (arteriolar) and vessel leakiness induce circulatory shock. Bronchial spasms cause respiratory insufficiency; combined effect is anaphylactic shock. 5. Similarity to tissue mast cells: Tissue mast cells also have IgE receptors and similar (though not identical) granule content. Mast cells and basophils have a common precursor in bone marrow. Specific granules • Histamine • Heparin • Eosinophil chemotactic factor • Phospholipids for synthesis of leukotrienes, e.g. slow-reacting substance of anaphylaxis ( SRS-A ) Non-specific granules (lysosomes) • Lysozyme • Acid hydrolase • Myeloperoxidase • Elastase
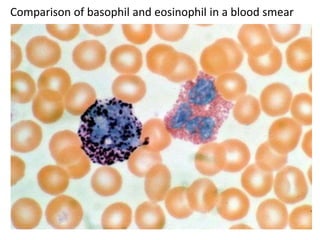
- 14. Comparison of basophil and eosinophil in a blood smear
- 15. Lymphocyte 1. Life Span: variable (few days to several years) 2. Small lymphocyte (about 90% of lymphocytes you will see) are ~8 µm in diameter, while large lymphocytes may be up to about 15 µm. Round, dense nucleus (abundant heterochromatin). The cytoplasm of a small lymphocyte is a narrow rim around the nucleus, and when well stained is pale blue. T-lymphocytes and B-lymphocytes cannot be distinguished in a smear. 3. The cytoplasm doesn't appear to be very active, containing mainly mitochondria and free ribosomes. 4. Function: Cellular and humoral immunity. In general: – B-lymphocytes (B-cells): may differentiate into tissue plasma B cells which make antibodies. Some B-cells become memory cells. – T-lymphocytes (T-cells): cytotoxic T cells and helper T cells.
- 16. Small lymphocyte in a blood smear Small lymphocyte
- 17. Large lymphocyte in a blood smear Large lymphocyte
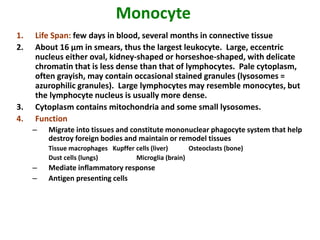
- 18. Monocyte 1. Life Span: few days in blood, several months in connective tissue 2. About 16 µm in smears, thus the largest leukocyte. Large, eccentric nucleus either oval, kidney-shaped or horseshoe-shaped, with delicate chromatin that is less dense than that of lymphocytes. Pale cytoplasm, often grayish, may contain occasional stained granules (lysosomes = azurophilic granules). Large lymphocytes may resemble monocytes, but the lymphocyte nucleus is usually more dense. 3. Cytoplasm contains mitochondria and some small lysosomes. 4. Function – Migrate into tissues and constitute mononuclear phagocyte system that help destroy foreign bodies and maintain or remodel tissues Tissue macrophages Kupffer cells (liver) Osteoclasts (bone) Dust cells (lungs) Microglia (brain) – Mediate inflammatory response – Antigen presenting cells
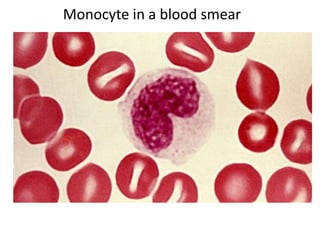
- 19. Monocyte in a blood smear
- 20. Major functions of WBCs • Defensive action: – Phagocytosis: The neutrophils and monocytes engulf foreign particles and microorganism. – Antibody formation: Lymphocytes produce antibodies (mainly gamma globulin) and play an important role in defensive mechanism of the body. • Secretion of heparin: The basophils secret heparin, which helps in the prevention of intravascular clot. • Manufacture of trephones: Leukocytes produce some special protein like substance known as “trephones” which have great influence on nutrition, growth and tissue repair .
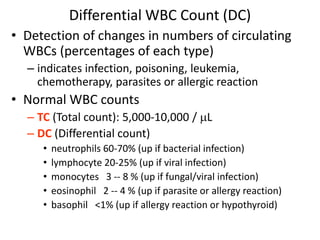
- 21. Differential WBC Count (DC) • Detection of changes in numbers of circulating WBCs (percentages of each type) – indicates infection, poisoning, leukemia, chemotherapy, parasites or allergic reaction • Normal WBC counts – TC (Total count): 5,000-10,000 / L – DC (Differential count) • neutrophils 60-70% (up if bacterial infection) • lymphocyte 20-25% (up if viral infection) • monocytes 3 -- 8 % (up if fungal/viral infection) • eosinophil 2 -- 4 % (up if parasite or allergy reaction) • basophil <1% (up if allergy reaction or hypothyroid)
- 22. Inflammation: Role of Neutrophils and Macrophages When tissue injury occurs, whether caused by bacteria, trauma, chemicals, heat, or any other phenomenon, multiple substances are released by the injured tissues and cause dramatic secondary changes in the surrounding uninjured tissues. This entire complex of tissue changes is called inflammation. “Walling-Off” Effect of Inflammation. One of the first results of inflammation is to “wall off ” the area of injury from the remaining tissues. The tissue spaces and the lymphatics in the inflamed area are blocked by fibrinogen clots so that after a while, fluid barely flows through the spaces. This walling-off process delays the spread of bacteria or toxic products. Tissue Macrophage Is a First Line of Defense Against Infection. Within minutes after inflammation begins, the macrophages already present in the tissues, whether histiocytes in the subcutaneous tissues, alveolar macrophages in the lungs, microglia in the brain, or others, immediately begin their phagocytic actions. Neutrophil Invasion of the Inflamed Area Is a Second Line of Defense. Within the first hour or so after inflammation begins, large numbers of neutrophils begin to invade the inflamed area from the blood. Because the blood neutrophils are already mature cells, they are ready to immediately begin their scavenger functions for killing bacteria and removing foreign matter.
- 23. Acute Increase in Number of Neutrophils in the Blood—“Neutrophilia.” Also within a few hours after the onset of acute, severe inflammation, the number of neutrophils in the blood sometimes increases fourfold to fivefold—from a normal of 4000 to 5000 to 15,000 to 25,000 neutrophils per microliter. This is called neutrophilia, which means an increase in the number of neutrophils in the blood. Second Macrophage Invasion into the Inflamed Tissue Is a Third Line of Defense. Along with the invasion of neutrophils, monocytes from the blood enter the inflamed tissue and enlarge to become macrophages. Even after invading the inflamed tissue, monocytes are still immature cells, requiring 8 hours or more to swell to much larger sizes and develop tremendous quantities of lysosomes; only then do they acquire the full capacity of tissue macrophages for phagocytosis. As already pointed out, macrophages can phagocytize far more bacteria (about five times as many) and far larger particles, including even neutrophils themselves and large quantities of necrotic tissue, than can neutrophils. Increased Production of Granulocytes and Monocytes by the Bone Marrow Is a Fourth Line of Defense. The fourth line of defense is greatly increased production of both granulocytes and monocytes by the bone marrow. This results from stimulation of the granulocytic and monocytic progenitor cells of the marrow. However, it takes 3 to 4 days before newly formed granulocytes and monocytes reach the stage of leaving the bone marrow.
- 24. Formation of Pus When neutrophils and macrophages engulf large numbers of bacteria and necrotic tissue, essentially all the neutrophils and many, if not most, of the macrophages eventually die. After several days, a cavity is often excavated in the inflamed tissues that contains varying portions of necrotic tissue, dead neutrophils, dead macrophages, and tissue fluid. This mixture is commonly known as pus.
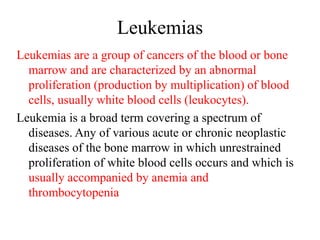
- 25. Leukemias Leukemias are a group of cancers of the blood or bone marrow and are characterized by an abnormal proliferation (production by multiplication) of blood cells, usually white blood cells (leukocytes). Leukemia is a broad term covering a spectrum of diseases. Any of various acute or chronic neoplastic diseases of the bone marrow in which unrestrained proliferation of white blood cells occurs and which is usually accompanied by anemia and thrombocytopenia
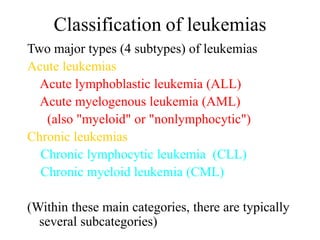
- 26. Classification of leukemias Two major types (4 subtypes) of leukemias Acute leukemias Acute lymphoblastic leukemia (ALL) Acute myelogenous leukemia (AML) (also "myeloid" or "nonlymphocytic") Chronic leukemias Chronic lymphocytic leukemia (CLL) Chronic myeloid leukemia (CML) (Within these main categories, there are typically several subcategories)
- 27. Myeloid vs Lymphoid • Any disease that arises from the myeloid elements (white cell, red cell, platelets) is a myeloid disease ….. AML, CML • Any disease that arises from the lymphoid elements is a lymphoid disease ….. ALL, CLL
- 28. Acute vs. chronic leukemia • Acute leukemias: • Young, immature, blast cells in the bone marrow (and often blood) • More fulminant (severe and sudden in onset) presentation • More aggressive course • Chronic leukemias: • Accumulation of mature, differentiated cells • Often subclinical or incidental presentation • In general, more indolent (slow) course • Frequently splenomegaly • Mature appearing cells in the B. marrow and blood
- 29. Leukopenia A clinical condition known as leukopenia occasionally occurs in which the bone marrow produces very few white blood cells, leaving the body unprotected against many bacteria and other agents that might invade the tissues. Within 2 days after the bone marrow stops producing white blood cells, ulcers may appear in the mouth and colon, or the person might develop some form of severe respiratory infection. Bacteria from the ulcers rapidly invade surrounding tissues and the blood. Without treatment, death often ensues in less than a week after acute total leukopenia begins. Irradiation of the body by x-rays or gamma rays, or exposure to some drugs and chemicals, is likely to cause aplasia of the bone marrow.
- 30. THANK YOU