Amylin
- 2. Outlines • Introduction • Functions • Metabolism • Amyloid formation • Therapeutic applications 2
- 3. Amylin or Islet Amyloid Polypeptide (IAPP) A neuroendocrine hormone 37-amino acid peptide first reported in 1987 Co-localized and co-secreted with insulin from pancreatic b-cells actions as a hormone, growth factor, and modifier of behavior Deficient in diabetes Amylin InsulinHuman amylin 3
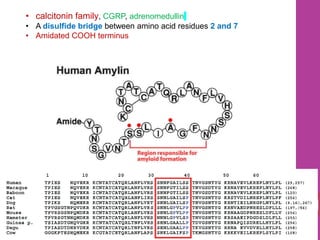
- 4. • calcitonin family, CGRP, adrenomedullin • A disulfide bridge between amino acid residues 2 and 7 • Amidated COOH terminus 4
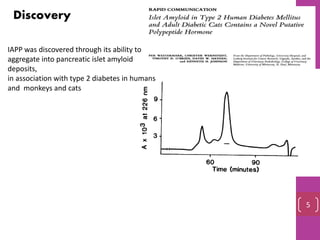
- 5. Discovery IAPP was discovered through its ability to aggregate into pancreatic islet amyloid deposits, in association with type 2 diabetes in humans and monkeys and cats 5
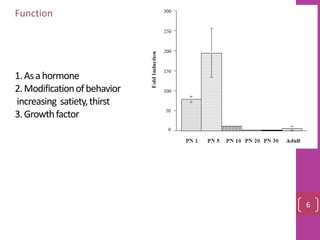
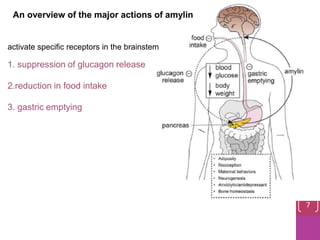
- 7. An overview of the major actions of amylin activate specific receptors in the brainstem 1. suppression of glucagon release 2.reduction in food intake 3. gastric emptying 7
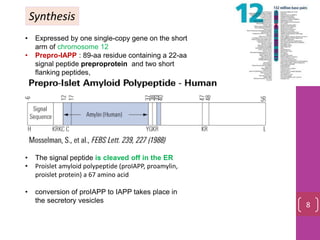
- 8. Synthesis • Expressed by one single-copy gene on the short arm of chromosome 12 • Prepro-IAPP : 89-aa residue containing a 22-aa signal peptide preproprotein and two short flanking peptides, • The signal peptide is cleaved off in the ER • Proislet amyloid polypeptide (proIAPP, proamylin, proislet protein) a 67 amino acid • conversion of proIAPP to IAPP takes place in the secretory vesicles 8
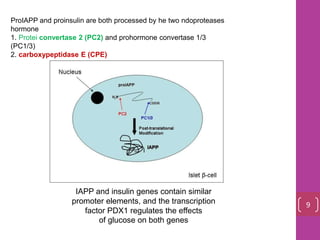
- 9. ProIAPP and proinsulin are both processed by he two ndoproteases hormone 1. Protei convertase 2 (PC2) and prohormone convertase 1/3 (PC1/3) 2. carboxypeptidase E (CPE) IAPP and insulin genes contain similar promoter elements, and the transcription factor PDX1 regulates the effects of glucose on both genes 9
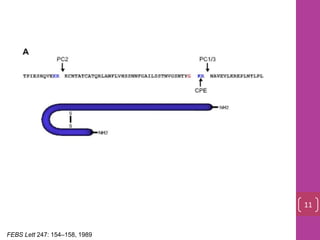
- 10. FEBS Lett 247: 154–158, 1989 11
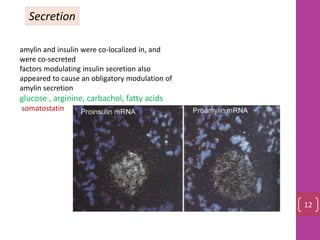
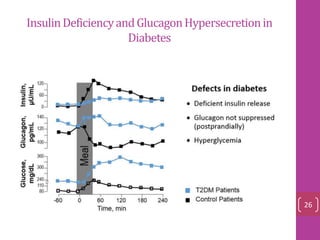
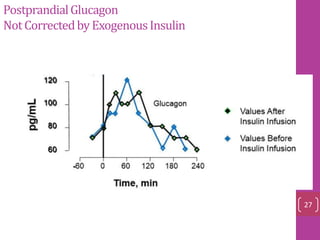
- 11. Secretion amylin and insulin were co‐localized in, and were co‐secreted factors modulating insulin secretion also appeared to cause an obligatory modulation of amylin secretion glucose , arginine, carbachol, fatty acids somatostatin 12
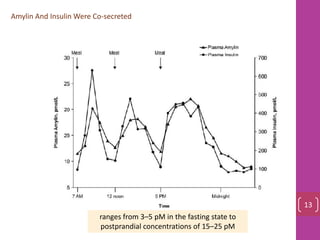
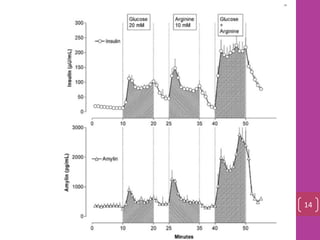
- 12. Amylin And Insulin Were Co‐secreted ranges from 3–5 pM in the fasting state to postprandial concentrations of 15–25 pM 13
- 13. 14
- 14. • Like the C peptide, IAPP is eliminated in the kidney • Insulin-degrading enzyme (IDE,Insulysin and insulinase) • A second protease capable of degrading amyloid is neprolysin Metabolism 15
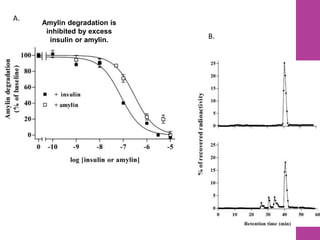
- 15. Amylin degradation is inhibited by excess insulin or amylin. A. B. 16
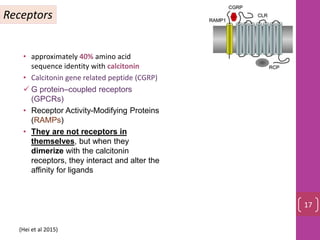
- 16. • approximately 40% amino acid sequence identity with calcitonin • Calcitonin gene related peptide (CGRP) G protein–coupled receptors (GPCRs) • Receptor Activity-Modifying Proteins (RAMPs) • They are not receptors in themselves, but when they dimerize with the calcitonin receptors, they interact and alter the affinity for ligands Receptors 17 (Hei et al 2015)
- 17. Receptor activity-modifying proteins (RAMPs) are a class of protein that interact with and modulate the activities of several Class B G Protein-Coupled Receptors including the receptors for calcitonin(CT), glucagon,and vasoactive intestinal peptide (VIP). There are three distinct types of RAMPs, designated RAMP1, RAMP2, and RAMP3, each encoded by a separate gene 18
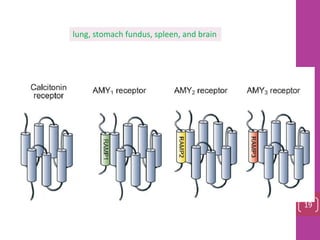
- 18. lung, stomach fundus, spleen, and brain 19
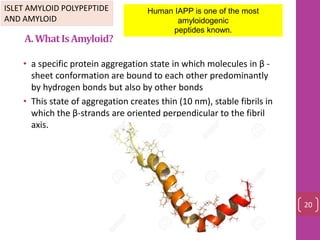
- 19. A.WhatIsAmyloid? • a specific protein aggregation state in which molecules in β - sheet conformation are bound to each other predominantly by hydrogen bonds but also by other bonds • This state of aggregation creates thin (10 nm), stable fibrils in which the β-strands are oriented perpendicular to the fibril axis. ISLET AMYLOID POLYPEPTIDE AND AMYLOID Human IAPP is one of the most amyloidogenic peptides known. 20
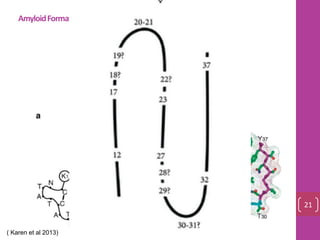
- 20. AmyloidFormation 21 ( Karen et al 2013)
- 21. 22
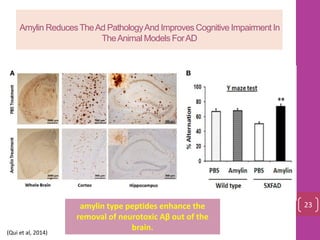
- 22. Amylin ReducesTheAd PathologyAnd Improves Cognitive Impairment In TheAnimal Models ForAD amylin type peptides enhance the removal of neurotoxic Aβ out of the brain. 23 (Qui et al, 2014)
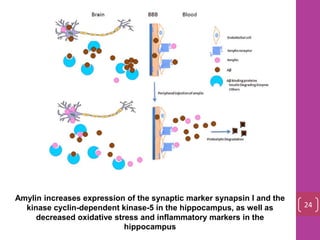
- 23. Amylin increases expression of the synaptic marker synapsin I and the kinase cyclin-dependent kinase-5 in the hippocampus, as well as decreased oxidative stress and inflammatory markers in the hippocampus 24
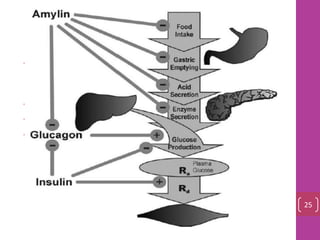
- 24. Glucose Homeostasis • Amylin is released during the feeding/fed state in response to nutrient entry into the gastrointestinal tract • appetite • glucagon • Gastric empting 25
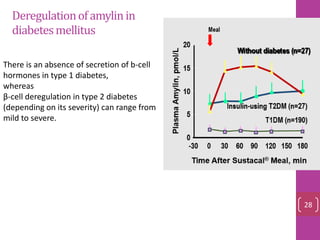
- 27. Deregulationofamylinin diabetesmellitus There is an absence of secretion of b-cell hormones in type 1 diabetes, whereas β-cell deregulation in type 2 diabetes (depending on its severity) can range from mild to severe. 28
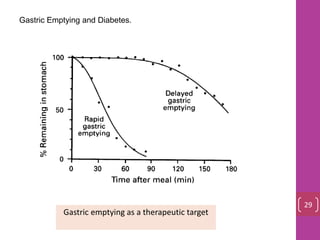
- 28. Gastric Emptying and Diabetes. Gastric emptying as a therapeutic target 29
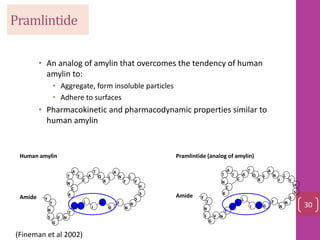
- 29. Pramlintide • An analog of amylin that overcomes the tendency of human amylin to: • Aggregate, form insoluble particles • Adhere to surfaces • Pharmacokinetic and pharmacodynamic properties similar to human amylin Human amylin Pramlintide (analog of amylin) Amide S S A Y T N S G V N T T T T N A A A L I K S S C C Q R L N N NF G F L V H Amide P P P Y T N S G V N T T T T N A A A L I K S S C C Q R L N N NF G F L V H 30 (Fineman et al 2002)
- 30. PramlintideMimickedThree Important Actions of AmylinThat ImpactGlucose Appearance Amylin* Pramlintide Slows gastric emptying Promotes satiety and reduces caloric intake Inhibits inappropriately high postprandial glucagon secretion 31
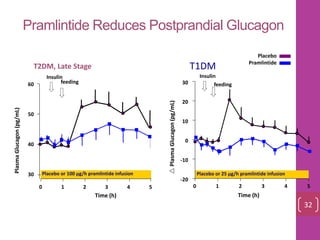
- 31. Pramlintide Reduces Postprandial Glucagon T1DM Time (h) Placebo Pramlintide Placebo or 25 µg/h pramlintide infusion -20 0 10 20 30 -10 Insulin 0 2 3 4 51 T2DM, Late Stage Time (h) PlasmaGlucagon(pg/mL) Insulin 60 40 30 50 Placebo or 100 µg/h pramlintide infusion 0 1 2 3 4 5 PlasmaGlucagon(pg/mL) 32 feedingfeeding
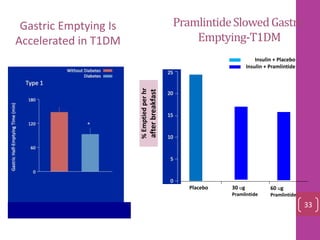
- 32. %Emptiedperhr afterbreakfast Placebo 30 ug Pramlintide 60 ug Pramlintide PramlintideSlowedGastric Emptying-T1DM Insulin + Placebo Insulin + Pramlintide Gastric Emptying Is Accelerated in T1DM 33
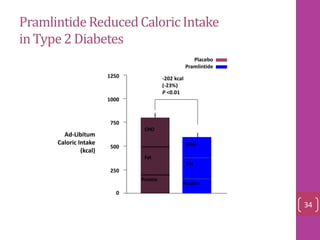
- 33. PramlintideReducedCaloric Intake in Type2 Diabetes 0 250 500 750 1000 1250 Protein CHO Fat CHO Fat Protein -202 kcal (-23%) P <0.01 Ad-Libitum Caloric Intake (kcal) Placebo Pramlintide 34
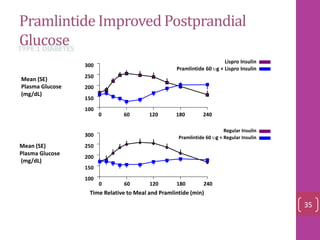
- 34. Pramlintide Improved Postprandial Glucose 100 150 200 250 300 0 60 120 180 240 Time Relative to Meal and Pramlintide (min) Mean (SE) Plasma Glucose (mg/dL) 100 150 200 250 300 0 60 120 180 240 Mean (SE) Plasma Glucose (mg/dL) Lispro Insulin Pramlintide 60 ug + Lispro Insulin Regular Insulin Pramlintide 60 ug + Regular Insulin TYPE 1 DIABETES 35
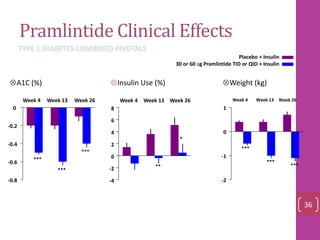
- 35. Pramlintide Clinical Effects -0.8 -0.6 -0.4 -0.2 0 -4 -2 0 2 4 6 8 -2 -1 0 1 *** *** *** ** * *** *** *** Week 4 Week 13 Week 26Week 4 Week 13 Week 26Week 4 Week 13 Week 26 Insulin Use (%)A1C (%) Weight (kg) Placebo + Insulin 30 or 60 ug Pramlintide TID or QID + Insulin TYPE 1 DIABETES COMBINED PIVOTALS 36
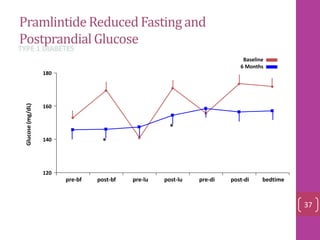
- 36. PramlintideReducedFastingand PostprandialGlucose 120 140 160 180 pre-bf post-bf pre-lu post-lu pre-di post-di bedtime Glucose(mg/dL) Baseline 6 Months * * TYPE 1 DIABETES 37
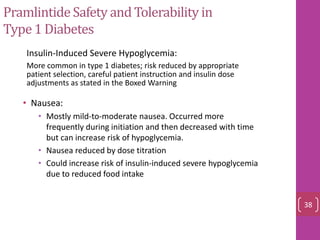
- 37. PramlintideSafety and Tolerability in Type1 Diabetes • Nausea: • Mostly mild-to-moderate nausea. Occurred more frequently during initiation and then decreased with time but can increase risk of hypoglycemia. • Nausea reduced by dose titration • Could increase risk of insulin-induced severe hypoglycemia due to reduced food intake • Insulin-Induced Severe Hypoglycemia: – More common in type 1 diabetes; risk reduced by appropriate patient selection, careful patient instruction and insulin dose adjustments as stated in the Boxed Warning 38
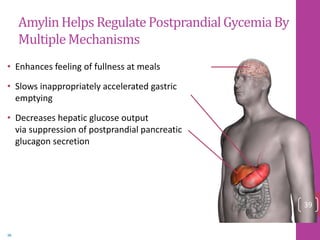
- 38. AmylinHelps Regulate PostprandialGycemiaBy MultipleMechanisms • Enhances feeling of fullness at meals • Slows inappropriately accelerated gastric emptying • Decreases hepatic glucose output via suppression of postprandial pancreatic glucagon secretion 39 Young A. Adv Pharmacol. 2005;52:67-77. 39
- 39. 40
- 40. 1. Hay, D.L., et al., Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev, 2015. 67(3): p. 564-600. 2. Westermark, P., A. Andersson, and G.T. Westermark, Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiological reviews, 2011. 91(3): p. 795-826. 3. Westermark, P., et al., A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochemical and biophysical research communications, 1986. 140(3): p. 827-831. 4. Woods, S.C., et al., Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 2006. 361(1471): p. 1219-1235. 5. Marzban, L., et al., Erratum. Small Interfering RNA-Mediated Suppression of Proislet Amyloid Polypeptide Expression Inhibits Islet Amyloid Formation and Enhances Survival of Human Islets in Culture. Diabetes 2008;57:3045-3055. Diabetes, 2016. 65(3): p. 818. 6. Lee, S.M., D.L. Hay, and A.A. Pioszak, Calcitonin and Amylin Receptor Peptide Interaction Mechanisms: Insights into Peptide-binding Modes and Allosteric Modulation of the Calcitonin Receptor by Receptor Activity-modifying Proteins. J Biol Chem, 2016. References 41
- 41. • 7. Ilitchev, A.I., et al., Human Islet Amyloid Polypeptide N-Terminus Fragment Self-Assembly: Effect of Conserved Disulfide Bond on Aggregation Propensity. J Am Soc Mass Spectrom, 2016. • 8. Bower, R.L., et al., Mapping the calcitonin receptor in human brainstem. Am J Physiol Regul Integr Comp Physiol, 2016: p. ajpregu.00539.2015. • 9. Van Hulst, K., et al., Islet amyloid polypeptide/amylin messenger RNA and protein expression in human insulinomas in relation to amyloid formation. European journal of endocrinology, 1999. 140(1): p. 69-78. • 10. Middleton, C.T., et al., Two-dimensional infrared spectroscopy reveals the complex behaviour of an amyloid fibril inhibitor. Nature chemistry, 2012. 4(5): p. 355- 360. • 11. Westermark, P., A. Andersson, and G.T. Westermark, Is aggregated IAPP a cause of beta-cell failure in transplanted human pancreatic islets? Current diabetes reports, 2005. 5(3): p. 184-188. • 12. Goldsbury, C., et al., Amyloid fibril formation from full-length and fragments of amylin. Journal of structural biology, 2000. 130(2): p. 352-362. 42
- 42. • 13. Drucker, D.J., The role of gut hormones in glucose homeostasis. The Journal of clinical investigation, 2007. 117(1): p. 24-32. • 14. Lutz, T.A., The role of amylin in the control of energy homeostasis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2010. 298(6): p. R1475-R1484. • 15. Higham, C.E., et al., Processing of synthetic pro‐islet amyloid polypeptide (proIAPP)‘amylin’by recombinant prohormone convertase enzymes, PC2 and PC3, in vitro. European Journal of Biochemistry, 2000. 267(16): p. 4998-5004. • 16. Hay, D., et al., Amylin receptors: molecular composition and pharmacology. Biochemical Society Transactions, 2004. 32(5): p. 865-867. • 17. Qi, D., et al., Fatty acids induce amylin expression and secretion by pancreatic β-cells. American Journal of Physiology-Endocrinology and Metabolism, 2010. 298(1): p. E99-E107. • 18. Wookey, P.J., et al., Amylin in the periphery. The Scientific World Journal, 2003. 3: p. 163-175. 43