DETECTORS USED IN GAS CHROMATOGRAPHY AND HPLC BY P.RAVISANKAR.
- 1. DETECTORS USED IN GASCHROMATOGRAPHY & HIGH PERFORMANCE LIQUID CHROMATOGRAPHY prof. Ravisankar Vignan Pharmacy college Valdlamudi Guntur Dist. Andhra Pradesh India. [email protected] 00919059994000
- 2. Contents: • Definition • Ideal properties of a detector • Detectors used in GC - concentration dependent detectors - mass flow dependent detectors • Detectors used in HPLC - selective detectors - universal detectors
- 3. Detectors: • The detector senses the presence of the individual components as they leave(elute) the column. The detector out put after amplification is traced on a recorder. • The duration of the intervals is usually a single second or even less than that. • Hence the detector is considered to be the brain of the instrument. • The detector converts a change in effluent into an electric signal that is recorded by data system
- 4. Ideal properties of a detector: The detectors used in both GC and HPLC should have following ideal properties: 1) High sensitivity. 2) Good stability and reproducibility. 3) A linear response to solutes. 4) Negligible base line noise. 5) Should be inexpensive. 6) Capable of providing information on the identity of solute. 7) A temperature range from room temperature to at least 4000c. 8) A short response time independent of flow rate. 9) High reliability and ease of operation. 10)The detector should be non-destructive. 11)Response independent of mobile phase composition.
- 5. Detectors used in GC: Detection devices for a GC must respond rapidly and reproducibility to the low concentrations of the solutes emitted from the column. Concentration dependent detectors: - Thermal conductivity detector(TCD) - Electron capture detector(ECD) - Argon ionization detector - Helium ionization detector Mass flow dependent detectors: - Flame ionization detector(FID) - Nitrogen phosphorous detector(NPD) - Flame photometric detector(FPD)
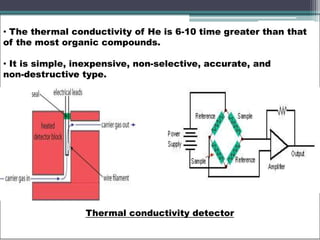
- 6. • In addition to these other detectors used are: - Thermionic detectors - Photoionization detectors - Atomic emission detectors - Sulfur chemiluminescence detector • The most widely used detectors are TCD, FID, ECD. Thermal conductivity detector(TCD): • TCD was one of the old detectors for GC, is still widely in use. • It is also known as katharometer and hot wire detector. • Principle in TCD is change in thermal conductivity of gas stream. • Thermal conductivity of most of the samples is lesser than most commonly used carrier gases like H, and He.
- 7. • The thermal conductivity of He is 6-10 time greater than that of the most organic compounds. • It is simple, inexpensive, non-selective, accurate, and non-destructive type. Thermal conductivity detector
- 8. • Since the detector response depends upon the difference in thermal conduction between sample and carrier gas, a large difference is essential. • An increase in temperature of the detector causes a change in the resistance of thermistor and this resistance gives a measure a measure of thermal conductivity of gas. • TCD consists of a temperature controlled metal block in which two cylindrical chambers are present, which consists of two filaments made up of platinum or tungsten. • Both the filaments are connected to the arms of Wheatstone bridge arrangement. • Resistance of filaments are constant as only the carrier gas is passed through them, once the effluent passes through them the change in conductivity is seen and is recorded.
- 9. Flame ionization detector(FID): • FID is the most widely used and generally applicable detector for GC. • With an FID the effluent from the column is directed into small air-hydrogen flame. • Most of the organic compounds produce ions and electrons when pyrolyzed at the temperature of air-hydrogen flame. • Detection invovles monitoring the current produced by collecting this charge carriers. • A few hundred volts applied between the burner tip and a collector electrode located above the flame causes the ions and electrons to move towards the collector. • The resulting current is then measured with high-impedance picoammeter.
- 10. • The ionization of carbon compounds in the FID is not fully understood, although the number of ions produced is roughly proportional to the number of reduced carbon atoms in flame. • Because the FID responds to the number of carbon atoms entering the detector per unit time. • The detector is insensitive towards non-combustible gases such as H2O, CO2, SO2, CO. • Functional groups like carbonyl, alcohol, halogen, and amine yield fewer ions or none at all in a flame. • These properties make the FID a most useful general detector for the analysis of most organic samples, including those contaminated with water and oxides of nitrogen and sulfur.
- 11. • The FID exhibits a high sensitivity (10-13g/s), large linear response range(107), and low noise. • It is generally rugged and easy to use Disadvantages: • It destroys sample. • It requires additional Gases and controllers.
- 12. Electron-capture detector(ECD): • It is most widely used detector for environmental samples because it selectively responds to halogen containing organic compounds. e.g.: pesticides, polychlorinated biphenyls • In ECD, the sample eluate from a column is passed over a radioactive β emitter, usually nickel-63. • The ECD is selective in its response. • An electron from the emitter causes ionization of carrier gas and the production burst of electrons. • In the absence of organic species, a constant standing current between a pair of electrodes results from this ionization. • In the presence of organic molecules containing electronegative functional groups that tends to capture electrons, hence the current decreases.
- 13. • Compounds such as halogens, peroxides, quinones and nitro groups are detected with high sensitivity. • The detector is insensitive to functional groups such as amines, alcohols and hydrocarbons. • An important application of ECD is for the detection and quantitative determination of chlorinated insecticides. • The advantage of ECD is that it does not alter the sample.
- 15. Flame photometric detector(FPD): • FPD is widely used in the analysis of air and water pollutants, pesticides, coal hydrogenation products • It is selective towards compounds containing sulfur and phosphorous. • In this detector the eluent is passed in to a low temperature hydrogen-air flame, which converts part of phosphorous in to HPO species that emits bands of radiation at about 510 and 526 nm. • It also converts sulfur in to S2 which emits a band at 394nm. • Suitable filters are used to isolate appropriate bands and their intensity is recorded photometrically. • FPD is used to detect elements like halogens, nitrogen and metals like tin, chromium, selenium and germanium.
- 17. Photoionization detector: • In this the molecules eluting from GC column are photoionized by ultra-violet radiation from a 10.2 eV hydrogen or a 11.7 eV argon lamp. • This source ionizes species with an ionization potential below the lamp energy. • compounds with a higher ionization potential do not absorb the energy and thus they are not detected. • The ions and electrons produced by Photoionization are then collected at pair of biased electrode. • The detector is most sensitive for aromatic hydrocarbons and organosulfur or organophosphorous.
- 19. Atomic emission detector(AED): • In AED, the effluent from GC column is introduced in to a microwave induced plasma(MIP), an inductively coupled plasma(ICP) or a direct current plasma. • Among these MIP has been most widely used and is available commercially. • The MIP is used in conjunction with diode array or charge coupled device atomic emission spectrometer. • The plasma is sufficiently energetic to atomize all of elements in a sample and to excite their characteristic atomic emission spectra. • Hence, the AED is an element selective detector.
- 20. • The sample in this case consists of gasoline containing small concentrations of methyl tertiary butyl ether(MTBE), as well as several aliphatic alcohols in low concentrations. • By using oxygen(777nm) rather than carbon(198nm) in lamp we can obtain a chromatogram peaks for alcohols and for MTBE which are readily identifiable.
- 21. Mass spectrometry detectors: • one of the most powerful detectors for GC is the mass spectrometer. • The combination of GC with Mass spectrometry is known as GC-MS. • Currently, nearly fifty instrument companies offer GC-MS equipment. • Currently, capillary columns are invariably used in GC-MS instruments and no other separators are needed. • Thermal degradation of components can be difficulty in GC- MS, lowering the temperature can minimize degradation. • However the mass spectrometer can be used to identify decomposition products, which can lead to chromatographic modifications that solve degradation problem.
- 22. • The most common ion sources used in GC-MS are electron- impact ionization and chemical ionization. • The most common mass analyzers are quadrapole and ion- trap analyzers. • In GC-MS, the mass spectrometer scans the masses repetitively during a chromatographic experiment. • GC-MS instruments have been used for identification of components present in natural and biological systems. e.g.: water pollutants, breath components and drug metabolites. • MS can also be used to obtain information about incompletely separated components. • It is extremely powerful tool for identifying components in the mixtures.
- 23. • Several other types of GC detectors are useful for detection of specific components, they are thermionic detectors and sulfur chemiluminescence detectors. • Thermionic detector is selective towards organic compounds containing phosphorous and nitrogen. • The response to a phosphorous atom is approximately ten times greater than a nitrogen atom and 104-106 times larger than to a carbon atom. • Sulfur chemiluminiscence detector is specific to the sulfur atom. • It is based on the reaction between certain sulfur compounds and ozone. • This detector has proven particularly useful for the determination of the pollutants such as mercaptans
- 24. Detector Type Support gases Selectivity Detectability Dynamic range Flame ionization (FID) Mass flow Hydrogen and air Most organic compounds. 100 pg 107 Thermal conductivity (TCD) Concentration Reference Universal 1 ng 107 Electron capture (ECD) Concentration Make-up Halides, nitrates, nitriles, peroxides, anhydrides, organometallics 50 fg 105 Nitrogen- phosphorus Mass flow Hydrogen and air Nitrogen, phosphorus 10 pg 106 Flame photometric (FPD) Mass flow Hydrogen and air possibly oxygen Sulphur, phosphorus, tin, boron, arsenic, germanium, selenium, chromium 100 pg 103 Photo-ionization (PID) Concentration Make-up Aliphatics, aromatics, ketones, esters, aldehydes, amines, heterocyclics, organosulphurs, some organometallics 2 pg 107 Hall electrolytic conductivity Mass flow Hydrogen, oxygen Halide, nitrogen, nitrosamine, sulphur - -
- 25. Detectors used in HPLC: • The function of the detector used in HPLC is to monitor the mobile phase it emerges from column. • The detectors used in HPLC are of majorly two types: selective detectors(solute property): • Absorbance detectors • Fluorescence detectors • Electrochemical detectors • Mass spectrometric detectors Universal detectors(bulk property): • Refractive index detectors • Evaporative light scattering detectors
- 27. UV-Visible Absorption Detectors: • In this a Z-shape flow through cell for absorption measurements on eluents from a chromatographic column. • To minimize extracolumn band broadening, the volume of such a cell should be kept as small as possible, typically 1-10µl • Many absorption detectors are double-beam devices in which one beam passes through eluent cell and other beam is reference beam. • Matched photoelectric detectors are used to compare the intensities of the two beams. • Single beam instruments are also used, were the intensity measurements of the solvent system are stored in a computer memory and used for the calculation of absorbance.
- 28. UV absorption detectors with filters: • These are used earlier with a mercury lamp as the source. • Most commonly, the intense line at 254nm was isolated by filters, by substitution of filters lines at 250, 313, 334, and 365nm can also be used. • Because of this reason, these type detectors are restricted as most of the organic and inorganic species exhibit broad absorption bands. UV absorption detectors with scanning capabilities: • A detector with scanning spectrophotometer with grating optics is most widely used. • Several operational modes can be chosen with these detectors.
- 29. • For example, the entire chromatogram can be obtained at single wavelength or different wavelengths can be chosen for a single peak. • When entire spectra are desired for identification purposes the flow of eluent can be stopped for a sufficient period to permit scanning the wavelength region of interest.
- 30. Fluorescence detectors: • Fluorescence detectors for HPLC are similar in design to the fluorometers and spectrofluorometers. • In most, fluorescence is observed by a photoelectric transducer located at 900 excitation of beam. • The simplest detectors use mercury excitation source and one or more filters to isolate a band of emitted radiation. • More sophisticated instruments use a xenon source and a grating monochromator to isolate the fluorescence radiation. • Laser-induced fluorescence is also used because of its sensitivity and selectivity. • An inherent advantage of these detectors is their high sensitivity, hence used in LC for separation and determination of components of samples that fluoresce.
- 31. • Fluorescent compounds can be obtained by treating with reagents that form fluorescent derivatives. • For example, dansylchloride forms fluorescent compounds with primary amines, secondary amines, amino acids and phenols, hence widely used for detection of amino acids in protein hydrolyzates. Fluorescent detector
- 32. Refractive-index detectors: • The ability of a compound or a solvent to deflect light provides a way to detect it. • RI is a measure of molecule’s ability to deflect light in a flowing mobile phase in a flow cell relative to a static mobile phase contained in a reference cell. • The amount of deflection is proportional to concentration. • The RI detector is considered as a universal detector but it is not very sensitive. • To achieve high sensitivity, in practice solvents are selected that have a very high or very low refraction index. • The detection limit is in the range of 10-6-10-8 g/ml.
- 34. Electrochemical detectors: • This is based on amperometry, voltammetry, coloumetry, and conductometry. • Electron transfer processes offer highly selective and sensitive method. • Easily adaptable for use with micro columns. • As background noise is dependent on mobile phase conditions, it is difficult to utilize these detectors with gradient elution separations. • It is of two types: Amperometric detection: • Fixed potential is applied to the electrode (glassy carbon) and a solute which will oxidize at that potential yields an output current
- 35. Coulometric detection: • 100% of the solute species is converted, which offers advantages of no mobile phase flow dependence on signal and absolute quantitation through Faraday’s law.
- 36. • ELSD can outperform traditional detectors when analysing non- chromophoric samples by HPLC. • Traditional HPLC detectors such as UV and RI have limited capabilities. • UV and RI are not compatible with a wide range of solvents RI detection is not gradient compatible • Different analytes produce different UV responses depending on their extinction co-efficient. • ELSDs can detect anything that is less volatile than the mobile phase • ELSD is universal and compatible with a wide range of solvents Evaporative Light Scattering Detector:
- 37. • Three steps are involved in detection: - nebulization - mobile phase evaporation - detection Nebulization: • Column effluent passes through nebulizer needle. • It mixes with the nitrogen gas. • Dispersion of droplets are formed. Mobile phase evaporation: • The above formed droplets are passed through a heating zone. • Mobile phase evaporates from the particles.
- 38. Detection: • Sample particles pass through an optical cell. • Sample particles interrupt laser beam and scattered light. • Photodiode detects the scattered light. • ELSD is an effective replacement or a perfect complement to existing LC detectors.
- 39. Conclusion: Detector is the key element that is present in any device that is used for the identification and estimation of any compound. It detects in a faster rate i.e. with in seconds hence it is considered as brain if the instrument. With out a detector no one can analyze the compound. Hence, it attains such an importance in the field of analysis.
- 40. References: • Instrumental analysis by Skoog, Holler, and Crouch. • Instrumental methods of chemical analysis by B. K. Sharma. • Pharmaceutical analysis by David G. Watson. • Principles of instrumental analysis by Douglas A. Skoog and Donald M. West. • Basic gas chromatography by Harold M. McNair and James M. Miller.
- 41. THANK YOU