Enzymes
- 1. 4th SEMESTER – BOTANY KARNATAKA UNIVERSITY, DHARWAD Modified from various internet resources by Dr. Jayakara Bhandary Associate Professor of Botany Government Arts & Science College Karwar, Uttara Kannada 1
- 2. Introduction & History Enzyme, in Greek means in living (en= in, zyme = living). Biocatalysts or Organic catalysts, usually high molecular weight proteins (exception- Ribozymes or RNA enzymes). Coined by Kuhne in 1878. First enzyme extract from Yeast cells by Buchner (1897). First purified enzyme is urease, by James B. Summer (1926). 2
- 3. Enzymes are Biological Catalysts Enzymes are proteins that: Increase the rate of reaction by lowering the energy of activation. Catalyze nearly all the chemical reactions taking place in the cells of the body. Have unique three- dimensional shapes that fit the shapes of reactants. 3
- 4. Activation Energy Think of activation energy as the BARRIER required to make a product. Most stable product is the one with the lowest energy. Most reactions require a “push” to get them started! “Push” is called “energy of activation” for reaction - Also represented by EA 4
- 5. Trivial Names of Enzymes The name of an enzyme: Usually ends in –ase. Identifies the reacting substance. For example, sucrase catalyzes the reaction of sucrose. Describes the function of the enzyme. For example, oxidases catalyze oxidation. Could be a common name, particularly for the digestion enzymes such as pepsin and trypsin. 5
- 6. IUB Classification of Enzymes Enzymes are classified according to the reaction they catalyze. Class Reactions catalyzed Oxidoreductases Oxidation-reduction Transferases Transfer groups of atoms Hydrolases Hydrolysis Lyases Add atoms/remove atoms to/from a double bond Isomerases Rearrange atoms Ligases Use ATP to combine molecules 6
- 7. Systematic Name According to the International union Of Biochemistry an enzyme name has two parts: -First part is the name of the substrates for the enzyme. -Second part is the type of reaction catalyzed by the enzyme.This part ends with the suffix “ase”. Example: Lactate dehydrogenase
- 8. EC number Enzymes are classified into six different groups according to the reaction being catalyzed. The nomenclature was determined by the Enzyme Commission in 1961 (with the latest update having occurred in 1992), hence all enzymes are assigned an “EC” number. The classification does not take into account amino acid sequence (ie, homology), protein structure, or chemical mechanism.
- 9. EC numbers EC numbers are four digits, for example a.b.c.d, where “a” is the class, “b” is the subclass, “c” is the sub- subclass, and “d” is the sub-sub-subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. Example: for Alcohol:NAD+oxidoreductase EC number is 1.1.1.1
- 10. The Six Classes EC 1. Oxidoreductases EC 2. Transferases EC 3. Hydrolases EC 4. Lyases EC 5. Isomerases EC 6. Ligases
- 11. EC 1. Oxidoreductases Catalyze the transfer of hydrogen or oxygen atoms or electrons from one substrate to another. Since these are ‘redox’ reactions, an electron donor/acceptor is also required to complete the reaction. A H2 +B → A+ BH2 Ex. Oxidases, Dehydrogenases, Reductases.
- 12. EC 2. Transferases Catalyze group transfer reactions, excluding oxidoreductases (which transfer hydrogen or oxygen and are EC 1). These are of the general form: A-X + B ↔ BX + A Ex: Transaminases (transfer amino group), Kinases (transfer Phosphate group)
- 13. EC 3. Hydrolases Catalyze hydrolytic reactions. Includes. A-X + H2O ↔ X-OH + A-H Ex: lipases, esterases, Amylases, peptidases/proteases, etc.
- 14. EC 4. Lyases Catalyze non-hydrolytic (covered in EC 3) removal of functional groups from substrates, often creating a double bond in the product; or the reverse reaction, ie, addition of function groups across a double bond. A- X +B-Y → A=B + X-Y Ex: Decarboxylases, Aldolases, Dehydrases, Deaminases, Synthases, etc.
- 15. EC 5. Isomerases Catalyzes isomerization reactions, including epimerizations and cis-trans isomerizations. A →A’ Ex: Isomerases (Cis-Trans), Epimerases (D—L)
- 16. EC 6. Ligases Catalyzes the synthesis of various (mostly C-X) bonds, coupled with the breakdown of energy- containing substrates, usually ATP . A+B → A-B ATP → ADP+iP Ex: Synthetases, Carboxylases
- 17. Classification of Enzymes: Oxidoreductases and Transferases 17
- 18. Classification: Hydrolases and Lyases 18
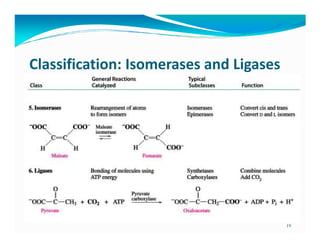
- 19. Classification: Isomerases and Ligases 19
- 20. Active Site The active site: Is a region within an enzyme that fits the shape of molecules called substrates. Contains amino acid R groups that align and bind the substrate. Releases products when the reaction is complete. 20
- 21. Enzyme Specificity Enzymes may recognize and catalyze: A single substrate. A group of similar substrates. A particular type of bond. 21
- 22. Mechanism of Enzyme Catalyzed Reactions The proper fit of a substrate (S) in an active site forms an enzyme-substrate (ES) complex. E+S ES Within the ES complex, the reaction occurs to convert substrate to product (P). ES E+P The products, which are no longer attracted to the active site, are released. Overall, substrate is convert to product. E+S ES E+P 22
- 23. Mechanism of Enzyme Action 23
- 24. 24
- 25. Example of An Enzyme Catalyzed Reaction 25
- 26. Mechanism of Enzyme Action: 1.Lock-and-Key Model In the lock-and-key model of enzyme action: The active site has a rigid shape. Only substrates with the matching shape can fit. The substrate is a key that fits the lock of the active site. 26
- 27. 2. Induced-fit Model In the induced-fit model of enzyme action: The active site is flexible, not rigid. The shapes of the enzyme, active site, and substrate adjust to maximum the fit, which improves catalysis. There is a greater range of substrate specificity. 27
- 28. Isoenzymes Isoenzymes catalyze the same reaction in different tissues in the body. Lactate dehydrogenase, which converts lactate to pyruvate, (LDH) consists of five isoenzymes. 28
- 29. Temperature and Enzyme Action Enzymes: Are most active at an optimum temperature (usually 37°C in humans). Show little activity at low temperatures. Lose activity at high temperatures as denaturation occurs. 29
- 30. pH and Enzyme Action Enzymes: Are most active at optimum pH. Contain R groups of amino acids with proper charges at optimum pH. Lose activity in low or high pH as tertiary structure is disrupted. 30
- 31. Optimum pH Values Most enzymes of the body have an optimum pH of about 7.4. In certain organs, enzymes operate at lower and higher optimum pH values. 31
- 32. Enzyme Concentration The rate of reaction increases as enzyme concentration increases (at constant substrate concentration). At higher enzyme concentrations, more substrate binds with enzyme. 32
- 33. Substrate Concentration The rate of reaction increases as substrate concentration increases (at constant enzyme concentration). Maximum activity occurs when the enzyme is saturated. 33