Coulometry
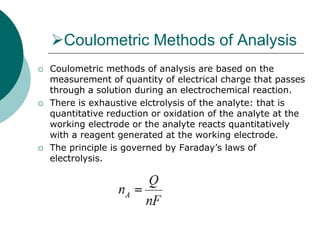
- 2. Coulometric Methods of Analysis Coulometric methods of analysis are based on the measurement of quantity of electrical charge that passes through a solution during an electrochemical reaction. There is exhaustive elctrolysis of the analyte: that is quantitative reduction or oxidation of the analyte at the working electrode or the analyte reacts quantitatively with a reagent generated at the working electrode. The principle is governed by Faraday’s laws of electrolysis.
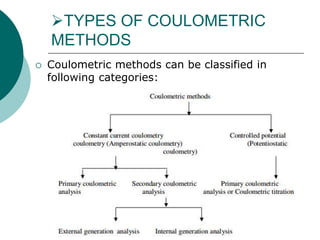
- 3. TYPES OF COULOMETRIC METHODS Coulometric methods can be classified in following categories:
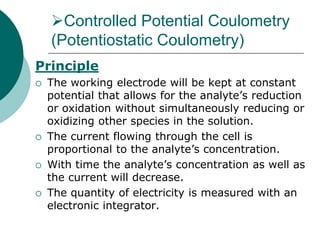
- 4. Controlled Potential Coulometry (Potentiostatic Coulometry) Principle The working electrode will be kept at constant potential that allows for the analyte’s reduction or oxidation without simultaneously reducing or oxidizing other species in the solution. The current flowing through the cell is proportional to the analyte’s concentration. With time the analyte’s concentration as well as the current will decrease. The quantity of electricity is measured with an electronic integrator.
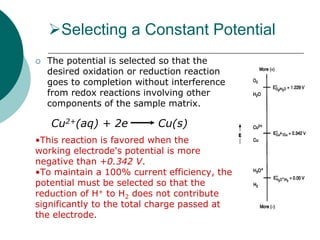
- 5. Selecting a Constant Potential The potential is selected so that the desired oxidation or reduction reaction goes to completion without interference from redox reactions involving other components of the sample matrix. •This reaction is favored when the working electrode's potential is more negative than +0.342 V. •To maintain a 100% current efficiency, the potential must be selected so that the reduction of H+ to H2 does not contribute significantly to the total charge passed at the electrode. Cu2+(aq) + 2e Cu(s)
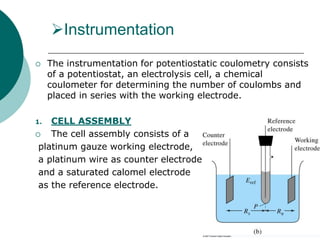
- 6. Instrumentation The instrumentation for potentiostatic coulometry consists of a potentiostat, an electrolysis cell, a chemical coulometer for determining the number of coulombs and placed in series with the working electrode. 1. CELL ASSEMBLY The cell assembly consists of a platinum gauze working electrode, a platinum wire as counter electrode and a saturated calomel electrode as the reference electrode.
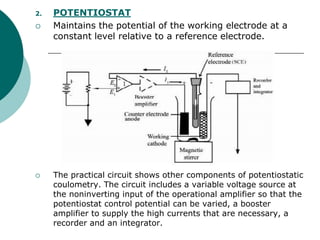
- 7. 2. POTENTIOSTAT Maintains the potential of the working electrode at a constant level relative to a reference electrode. The practical circuit shows other components of potentiostatic coulometry. The circuit includes a variable voltage source at the noninverting input of the operational amplifier so that the potentiostat control potential can be varied, a booster amplifier to supply the high currents that are necessary, a recorder and an integrator.
- 8. 3. INTEGRATORS Employed to determine the number of coulombs required to complete an electrolysis. Efficient stirring is important for controlled potential electrolysis, since all analyte species must be swept up to the electrode surface, so that the electrochemical reaction is completed. APPLICATIONS 1. Inorganic Analysis : Controlled potential coulometric methods have widespread use in the determination of several metal ions. As many as 55 elements of the periodic table can be determined by the cathodic reduction of metal ions to metallic state. Most of the can form amalgams with mercury, and hence controlled potential coulometry with mercury cathode is usually preferred.
- 9. 2. Micro analysis : Controlled potential coulometry is more popular than the electrogravimetric methods since it avoids the final step of weighing the product. This technique is especially useful for the determination of small amounts of analyte (0.01 – 1 mg) with an accuracy of (± 0.5 %). 3. Analysis of radioactive materials : The technique is widely adopted for the determination of uranium and plutonium and thus finds extensive use in the nuclear energy field. Reduction of UO2 2+ to U4+ can be carried out in H2SO4 medium with a mercury pool cathode (− 0.6 V vs. SCE). Samples containing 7 – 75 mg of uranium have been analyzed with an accuracy of ± 0.l %. 4. Electrolytic determination of organic compounds: Controlled potential coulometry offers a new step for the electrolytic determination of organic compounds. Trichloroacetic acid and picric acid are quantitatively reduced at a mercury cathode. Coulometric methods permit the analysis of these compounds with an accuracy of 0.1%.
- 10. References 1. Basic Concepts Of Analytical Chemistry, 2nd ed., S.M.Khopkar, New Age International Publishers. 2. Electrochemical methods: Fundamentals and Applications, 2nd ed., Allen J.Bard & Larry R.Faulkner, Wiley Publications. 3. Vogels Textbook Of Quantitative Chemical Analysis, 6th ed., Mendham, Denney, Barnes, Thomas, Pearson Education Ltd. 4. Principles of Instrumental Analysis, 5th ed., D.Skoog, J.Holler, T.Nieman, Saunders College Publication.