Buffer system
- 1. By Team B.N.Y.S S-VYASA
- 2. Contents…... INTRODUCTION ACIDS & BASES TYPES OF ACIDS & BASES pH BUFFERS TYPES OF BUFFER (MECHANISM) DISORDERS ROLE OF NUTRITION
- 3. Introduction Acid-base regulation – Regulation of hydrogen ion • Buffer system • Respiratory regulation • Renal regulation
- 4. Why To Regulate To maintain homeostasis Regulates enzymatic functions
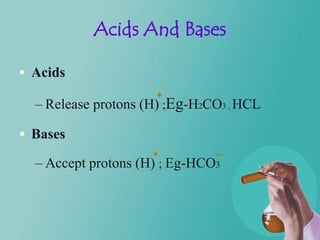
- 5. Acids And Bases Acids – Release protons (H) ;Eg-H2CO3 , HCL Bases – Accept protons (H) ; Eg-HCO3
- 6. Types of acids and bases Strong acids – Release large amount of Hydrogen ions Weak acids – Release small amount of Hydrogen ions Strong bases – Accept large amount of Hydrogen ions Weak bases – Accept small amount of Hydrogen ions
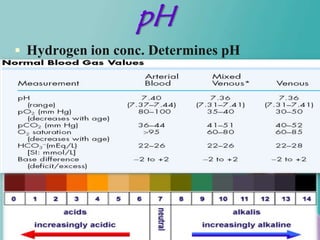
- 7. pH Hydrogen ion conc. Determines pH
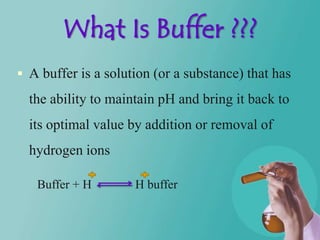
- 8. What Is Buffer ??? A buffer is a solution (or a substance) that has the ability to maintain pH and bring it back to its optimal value by addition or removal of hydrogen ions Buffer + H H buffer
- 9. Contd…. When Hydrogen ion conc. Increases – Reaction shifts towards right When Hydrogen ion conc. Decreases – Reaction shifts towards left In this way hydrogen ion concentration is maintained
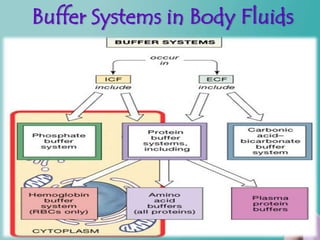
- 10. Types of chemical buffer – Carbonic acid-bicarbonate – – Buffering changes caused by organic and fixed acids – Protein buffer system-Amino acids – Minor buffering system- – Phosphate –Buffer pH in the ICF
- 11. Buffer Systems in Body Fluids Figure 27.7
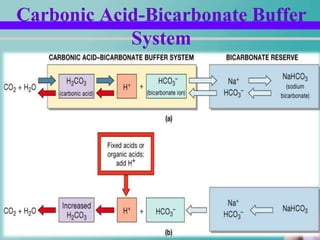
- 12. Carbonic Acid-Bicarbonate Buffering System Carbonic acid-bicarbonate buffer system Weak acid – H2CO3 Bicarbonate salt (NaHCO3) Strong acid is added – When HCL is added Hydrogen conc. increases – CO2 + H2O H2CO3 H + HCO3
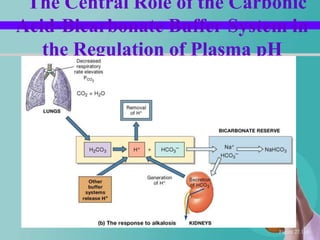
- 13. Contd… Strong base is added H+ conc. Reduces NaOH + H2CO3 NaHCO3+H2O In this way CO2 conc. decreases This inhibits respiration.
- 14. Carbonic Acid-Bicarbonate Buffer System
- 15. Bicarbonate buffer- Has the following limitations: – Cannot protect the ECF from pH changes due to increased or depressed CO2 levels – Only functions when respiratory system and control centers are working normally – It is limited by availability of bicarbonate ions (bicarbonate reserve).
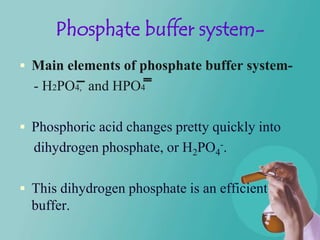
- 16. Phosphate buffer system- Main elements of phosphate buffer system- - H2PO4, and HPO4 Phosphoric acid changes pretty quickly into dihydrogen phosphate, or H2PO4-. This dihydrogen phosphate is an efficient buffer.
- 17. Contd…
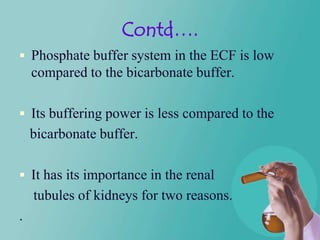
- 18. Contd…. Phosphate buffer system in the ECF is low compared to the bicarbonate buffer. Its buffering power is less compared to the bicarbonate buffer. It has its importance in the renal tubules of kidneys for two reasons. .
- 19. Contd 1.Conc. of phosphate is more in tubules. 2. Tubular fluid has lower pH. Conc. of phosphate is more in ICF compared to ECF.
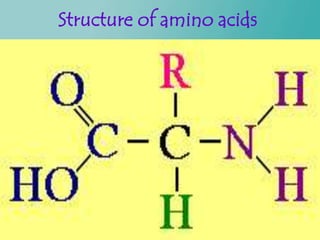
- 20. Protein buffer system Proteins are made up of amino acids Amino acids have a central carbon with four groups off of it: 1.a carboxyl group (COOH) 2.an amino group (NH2) 3.a hydrogen atom 4.an R group .
- 21. Structure of amino acids
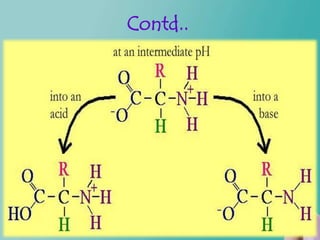
- 22. Contd… The carboxyl and amino groups are what enable proteins to act as buffers. Carboxyl group is attached to the amino acid central carbon: C - COOH Carboxyl group consists of a double bond to one of the oxygens and a single bond to the hydroxyl group.
- 23. Contd... At neutral pH the carboxyl ion is present as COO instead of COOH. Acidic medium – becomes COOH Basic medium – becomes COO.
- 24. Contd… Amino group is attached to the amino acid central carbon: C - NH2. Neutral pH, the amino group is actually- NH3+ rather than just NH2. Acidic medium – becomes NH3+ Basic medium- becomes NH2
- 25. Contd..
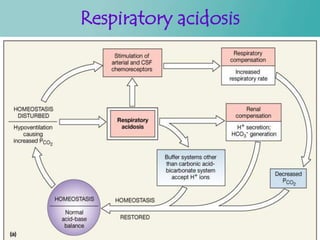
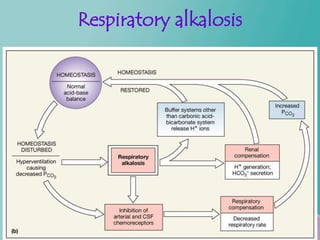
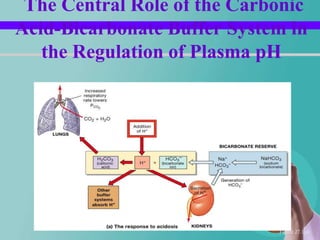
- 26. Respiratory regulation When alveolar ventilation increases CO2 conc. In ECF decreases H+ conc. decreases Or vice versa.......
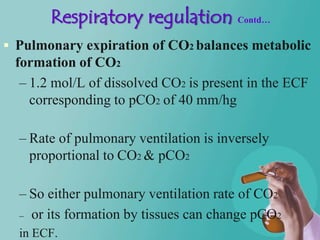
- 27. Respiratory regulation Contd… Pulmonary expiration of CO2 balances metabolic formation of CO2 – 1.2 mol/L of dissolved CO2 is present in the ECF corresponding to pCO2 of 40 mm/hg – Rate of pulmonary ventilation is inversely proportional to CO2 & pCO2 – So either pulmonary ventilation rate of CO2 – or its formation by tissues can change pCO2 in ECF.
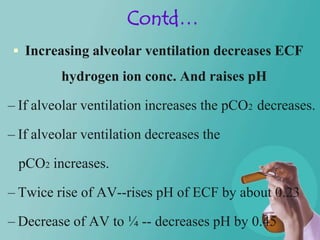
- 28. Contd… Increasing alveolar ventilation decreases ECF hydrogen ion conc. And raises pH – If alveolar ventilation increases the pCO2 decreases. – If alveolar ventilation decreases the pCO2 increases. – Twice rise of AV--rises pH of ECF by about 0.23 – Decrease of AV to ¼ -- decreases pH by 0.45
- 29. Contd… Increased Hydrogen ion conc. Stimulates alveolar ventilation Change in alveolar ventilation rate is much greater in reduced levels of pH than in increased levels of pH
- 30. Reason Alveolar ventilation rate decreases Increases pH O2 added in blood reduces Demand of O2 in blood increases pO2 also decreases Stimulates ventilation
- 31. Feedback control of Hydrogen ion conc. By RS H conc. Falls below normal Respiration is depressed Alveolar ventilation decreases H increases back to normal
- 32. Bufffering power of RS The kidneys work in elimination of hydrogen ion conc. and control imbalance. Its capacity is 1-2 times as much as other chemical buffers.
- 33. Impairment of lungs function: Impairment of lung function leads to emphysema and respiratory disorders. Kidneys play a major physiologic mechanism for returning pH to normal
- 34. Renal mechanism of acid-base regulation Kidneys regulate the blood pH by 1. maintaining alkali reserves 2. excreting / reabsorbing acid/base. Urine pH is lower than blood pH Kidneys- Acidification of urine.
- 35. Contd… Excretion of hydrogen ions Reabsorption of bicarbonate ion Excretion of ammonium ions
- 37. Disorders of acid-base regulation MAY LEAD TO--------- ALKALOSIS-- OR ACIDOSIS--
- 38. Disorders of acid-base regulation cont… Respiratory acidosis Respiratory alkalosis Metabolic acidosis Metabolic alkalosis
- 39. Contd… Respiratory acid-base disorders are initiated by an increase or decrease in partial pressure of carbondioxide whereas metabolic disorders are initiated by an increase or decrease in bicarbonate ion.
- 40. Contd… Alkalosis - Partial pressure of oxygen increases. Acidosis – Partial pressure of carbondioxide increases.
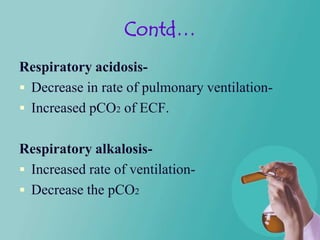
- 41. Contd… Respiratory acidosis- Decrease in rate of pulmonary ventilation- Increased pCO2 of ECF. Respiratory alkalosis- Increased rate of ventilation- Decrease the pCO2
- 42. Respiratory acidosis Figure 27.12a
- 43. Respiratory alkalosis Figure 27.12b
- 44. Contd… Metabolic acidosis- Decreased ECF bicarbonate ion conc. Metabolic alkalosis- Increased ECF bicarbonate ion conc.
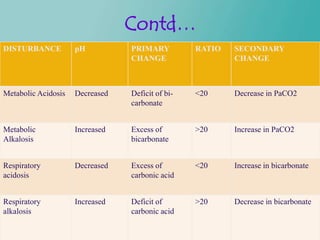
- 45. Contd… DISTURBANCE pH PRIMARY RATIO SECONDARY CHANGE CHANGE Metabolic Acidosis Decreased Deficit of bi- <20 Decrease in PaCO2 carbonate Metabolic Increased Excess of >20 Increase in PaCO2 Alkalosis bicarbonate Respiratory Decreased Excess of <20 Increase in bicarbonate acidosis carbonic acid Respiratory Increased Deficit of >20 Decrease in bicarbonate alkalosis carbonic acid
- 46. Contd… Causes - Metabolic acidosis 1. Renal tubular acidosis 2. Diarrhea 3. Vomiting of intestinal contents 4. Diabetes Mellitus 5. Ingestion of acids 6. Chronic renal failure
- 47. Contd… Causes- Metabolic alkalosis 1. Vomiting of gastric contents 2. Ingestion of alkaline drugs etc.
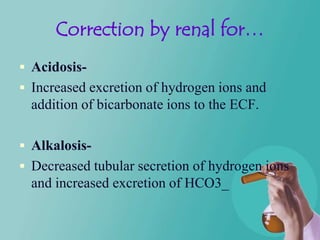
- 48. Correction by renal for… Acidosis- Increased excretion of hydrogen ions and addition of bicarbonate ions to the ECF. Alkalosis- Decreased tubular secretion of hydrogen ions and increased excretion of HCO3_
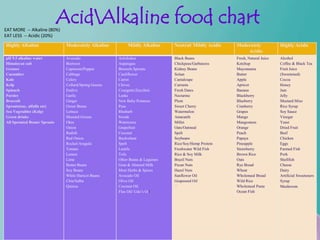
- 51. EAT MORE -- Alkaline (80%) AcidAlkaline food chart EAT LESS -- Acidic (20%) Highly Alkaline Moderately Alkaline Mildly Alkaline Neutral/ Mildly Acidic Moderately Highly Acidic Acidic pH 9.5 alkaline water Avocado Artichokes Black Beans Fresh, Natural Juice Alcohol Himalayan salt Beetroot Asparagus Chickpeas/Garbanzos Ketchup Coffee & Black Tea Grasses Capsicum/Pepper Brussels Sprouts Kidney Beans Mayonnaise Fruit Juice Cucumber Cabbage Cauliflower Seitan Butter (Sweetened) Kale Celery Carrot Cantaloupe Apple Cocoa Kelp Collard/Spring Greens Chives Currants Apricot Honey Spinach Endive Courgette/Zucchini Fresh Dates Banana Jam Parsley Garlic Leeks Nectarine Blackberry Jelly Broccoli Ginger New Baby Potatoes Plum Blueberry Mustard Miso Sprouts(soy, alfalfa etc) Green Beans Peas Sweet Cherry Cranberry Rice Syrup Sea Vegetables (Kelp) Lettuce Rhubarb Watermelon Grapes Soy Sauce Green drinks Mustard Greens Swede Amaranth Mango Vinegar All Sprouted Beans/ Sprouts Okra Watercress Millet Mangosteen Yeast Onion Grapefruit Oats/Oatmeal Orange Dried Fruit Radish Coconut Spelt Peach Beef Red Onion Buckwheat Soybeans Papaya Chicken Rocket/Arugula Spelt Rice/Soy/Hemp Protein Pineapple Eggs Tomato Lentils Freshwater Wild Fish Strawberry Farmed Fish Lemon Tofu Rice & Soy Milk Brown Rice Pork Lime Other Beans & Legumes Brazil Nuts Oats Shellfish Butter Beans Goat & Almond Milk Pecan Nuts Rye Bread Cheese Soy Beans Most Herbs & Spices Hazel Nuts Wheat Dairy White Haricot Beans Avocado Oil Sunflower Oil Wholemeal Bread Artificial Sweeteners Chia/Salba Olive Oil Grapeseed Oil Wild Rice Syrup Quinoa Coconut Oil ` Wholemeal Pasta Mushroom Flax Oil/ Udo’s Oil Ocean Fish
- 52. SO WHY NOT CONTROL THE ACID BASE BALANCE WITH FOOD…???
- 53. References Guyton's medical physiology,(Buffer System) Ionic changes By Rudolf Jensen
- 55. The Central Role of the Carbonic Acid-Bicarbonate Buffer System in the Regulation of Plasma pH Figure 27.11a
- 56. The Central Role of the Carbonic Acid-Bicarbonate Buffer System in the Regulation of Plasma pH Figure 27.11b
- 57. Acid-Base Disorders Respiratory acid base disorders – Result when abnormal respiratory function causes rise or fall in CO2 in ECF Metabolic acid-base disorders – Generation of organic or fixed acids – Anything affecting concentration of bicarbonate ions in ECF
- 58. Respiratory acidosis Results from excessive levels of CO2 in body fluids
- 59. Respiratory alkalosis Relatively rare condition Associated with hyperventilation
- 60. Metabolic acidosis Major causes are: – Depletion of bicarbonate reserve – Inability to excrete hydrogen ions at kidneys – Production of large numbers of fixed / organic acids – Bicarbonate loss due to chronic diarrhea
- 61. Figure 27.13 The Response to Metabolic Acidosis Figure 27.13
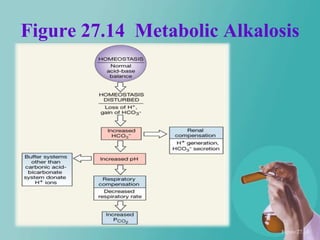
- 62. Figure 27.14 Metabolic Alkalosis Figure 27.14
- 63. Detection of acidosis and alkalosis Diagnostic blood tests – Blood pH – PCO2 – Bicarbonate levels Distinguish between respiratory and metabolic Animation: Acid-Base Homeostasis
- 64. Changes with age include Reduced total body water content Impaired ability to perform renal compensation Increased water demands – Reduced ability to concentrate urine – Reduced sensitivity to ADH/ aldosterone Net loss of minerals Inability to perform respiratory compensation Secondary conditions that affect fluid, electrolyte, acid- base balance
- 65. You should now be familiar with: What is meant by “fluid balance,” “electrolyte balance,” and “acid-base balance” The compositions of intracellular and extracellular fluids The hormones that play important roles in regulating fluid and electrolyte balance The movement of fluid that takes place within the ECF, between the ECF and the ICF, and between the ECF and the environment
- 66. You should now be familiar with: How sodium, potassium, calcium and chloride ions are regulated to maintain electrolyte balance The buffering systems that balance the pH of the intracellular and extracellular fluids The compensatory mechanisms involved in acid-base balance