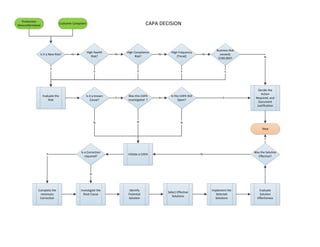
CAPA Decision
- 1. Production Nonconformance Customer Complaint Is it a New Risk? High Health Risk? N High Compliance Risk? N High Frequency (Trend) N N Business Risk exceeds $100,000? Stop N Evaluate the Risk Y Is it a known Cause? Y Y Y Y Was this CAPA investigated ? Y Decide the Action Required, and Document Justification Initiate a CAPA N Investigate the Root Cause Identify Potential Solution N Is a Correction required? Complete the necessary Correction Y Select Effective Solutions Implement the Selected Solutions Evaluate Solution Effectivness Was the Solution Effective? Y N N Y Is this CAPA Still Open? N Y CAPA DECISION