Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
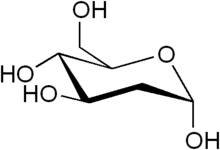
2-Deoxy-d-glucose is a glucose molecule which has the 2-hydroxyl group replaced by hydrogen, so that it cannot undergo further glycolysis. As such; it acts to competitively inhibit the production of glucose-6-phosphate from glucose at the phosphoglucoisomerase level (step 2 of glycolysis).[2] 2-Deoxyglucose labeled with tritium or carbon-14 has been a popular ligand for laboratory research in animal models, where distribution is assessed by tissue-slicing followed by autoradiography, sometimes in tandem with either conventional or electron microscopy.
 | |
| Names | |
|---|---|
| IUPAC name
2-Deoxy-D-arabino-hexopyranose | |
| Systematic IUPAC name
(4R,5S,6R)-6-(hydroxymethyl)oxane-2,4,5-triol | |
| Other names
2-Deoxyglucose 2-Deoxy-d-mannose 2-Deoxy-d-arabino-hexose 2-DG | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.295 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H12O5 | |
| Molar mass | 164.16 g/mol |
| Melting point | 142 to 144 °C (288 to 291 °F; 415 to 417 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-DG is up taken by the glucose transporters of the cell.[3] Therefore, cells with higher glucose uptake, for example tumor cells, have also a higher uptake of 2-DG. Since 2-DG hampers cell growth, its use as a tumor therapeutic has been suggested, and in fact, 2-DG is in clinical trials.[4] It is not completely clear how 2-DG inhibits cell growth. The fact that glycolysis is inhibited by 2-DG, seems not to be sufficient to explain why 2-DG treated cells stop growing.[5] A synergistic effect between 2-DG and various other agents have been reported in the pursuit of anticancer strategies.[6][7][8] Because of its structural similarity to mannose, 2DG has the potential to inhibit N-glycosylation in mammalian cells and other systems, and as such induces ER stress and the Unfolded Protein Response (UPR) pathway.[9][10][11]
2-DG has been used as a targeted optical imaging agent for fluorescent in vivo imaging.[12][13] In clinical medical imaging (PET scanning), fluorodeoxyglucose is used, where one of the 2-hydrogens of 2-deoxy-D-glucose is replaced with the positron-emitting isotope fluorine-18, which emits paired gamma rays, allowing distribution of the tracer to be imaged by external gamma camera(s). This is increasingly done in tandem with a CT function which is part of the same PET/CT machine, to allow better localization of small-volume tissue glucose-uptake differences.
On May 8, 2021, the Drugs Controller General of India approved an oral formulation of 2-deoxy-D-glucose for emergency use as adjunct therapy in moderate to severe coronavirus patients.[14][15] The drug was developed by the DRDO along with Dr. Reddy's Laboratories, who jointly claimed via a press release, that the drug "helps in faster recovery of hospitalised patients and reduces supplemental oxygen dependence".[15][16][17] The Wire as well as The Hindu noted that the approval was based on poor evidence; no journal publication (or preprint) concerning efficacy and safety are yet available.[16][17]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.